Chemical Properties of Asbestos
Chemical Properties of Asbestos
Chemical characteristics of Asbestos: solubility, effects of heat, acid resistance, + thermal insulation, emissivity
- POST a QUESTION or COMMENT about asbestos definition, chemical, mechanical, composition, iron content, thermal & other properties
Chemical properties of asbestos: this article describes the chemical properties of asbestos in various forms, such as asbestos solubility, the affect of acids on asbestos, the effects of heat on asbestos, and related properties.
This article series describes the physical properties of asbestos including its mechanical, chemical, electrical and related properties both in pure asbestos form and when asbestos is mixed with other materials like cement or rubber. As the author points out, while this is a lenghty article, there is far more detailed information about asbestos properties, chemistry, etc.
InspectAPedia tolerates no conflicts of interest. We have no relationship with advertisers, products, or services discussed at this website.
- Daniel Friedman, Publisher/Editor/Author - See WHO ARE WE?
Chemical Properties of Asbestos
One of the more important theories concerning formation of asbestos recognizes that the primary rock formation out of'which asbestos mother rock emerged is of volcanic origin. In Canada and other important asbestos areas, this green colored mother rock is principally olivine, classified chemically as magnesium silicate.
The pH of asbestos is generally listed as 9 to 10. When asbestos is acid washed or subjected to other chemicals, it can be made to behave tisfctôrily with acid curing poly mers or resin systems
The chemical feature common to all asbestos is that they are hydrated silicates The degree of hydration varies from approximately one per cent in some types to as much as approximately 14 per cent in most kinds of chiysotile It generally accepted that asbestos is a metamorphic product derived from certain types of silica-bearing minerals.
Effects of Chemicals on Asbestos
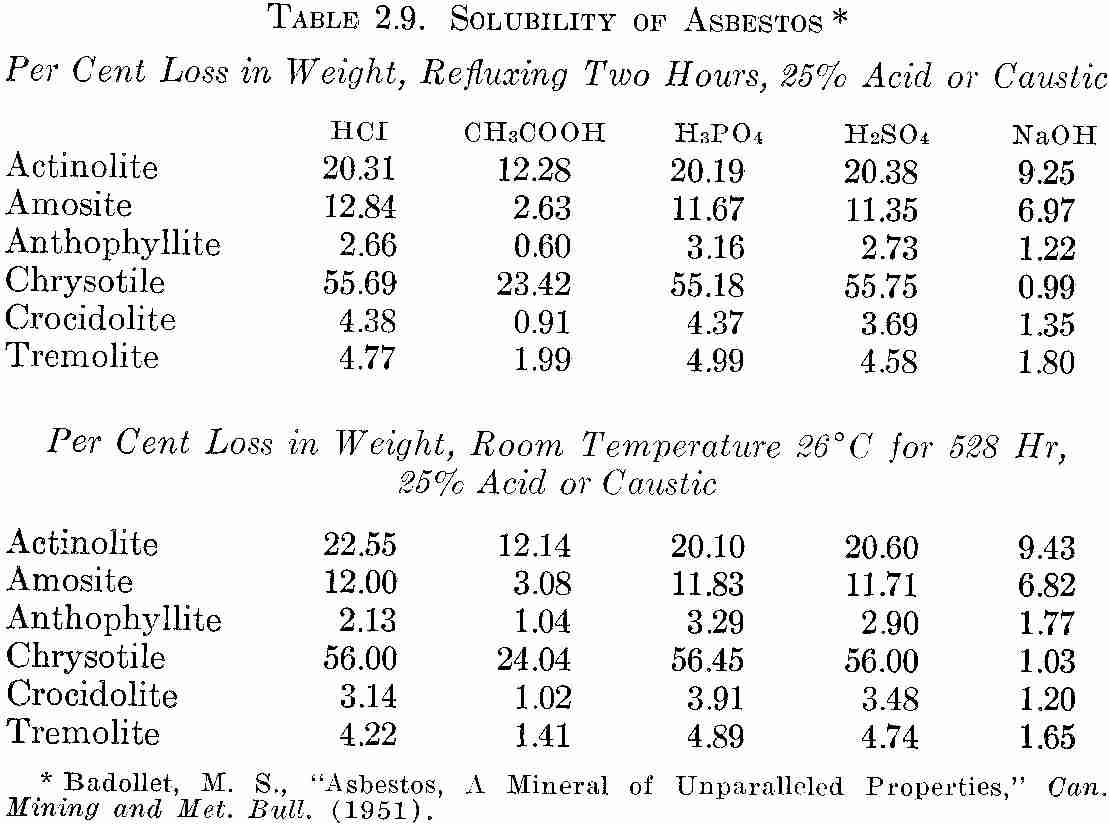
Published data showing the effect of chemicals on asbestos are given inTable 2.9 below. it is reported that room temperature tests were conducted for periods of 24, 192, 360, and 528 hr. * ASTM (D577-52, Method D39, Section 10) tensile grab test.
Inasmuch as most of the fibers reached maximum solubility after 528 hr, only this period was reported.
TABLE 2.9. SOLUBILITY OF ASBESTOS
[Click to enlarge]
Per Cent Loss in Weight, Refluxing Two Hours, 25% Acid or Caustic
* Badollet, M. S., "Asbestos, A Mineral of Unparalleled Properties," Can. Mining and Met. Bull. (1951).
Effects of Heat on Asbestos
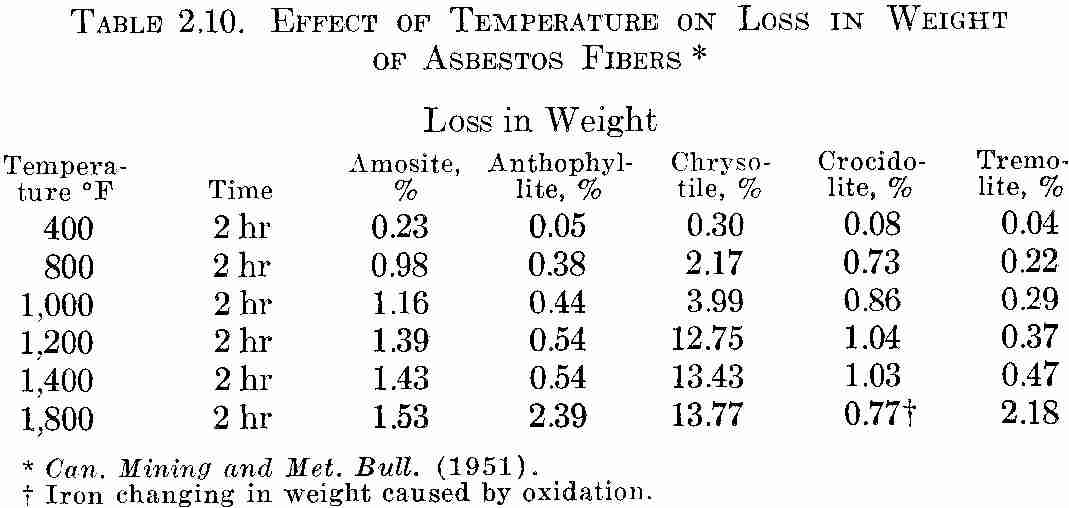
When asbestos is exposed to heat, it loses water of crystallization. At approximately 800°F, hornblende asbestos generally loses the greatest part of its combined water while serpentine loses only approximately 15 per cent. Hornblende asbestos becomes extremely brittle at 750°F.
In the case of serpentine asbestos, flexibility remains. Table 2.10 below gives the percentages of loss in weight versus temperatures up to 1,800°F. Test specimens were predried to remove surface water and weighed; After heat exposure for two hours, the asbestos was cooled at room temperature in a desiccator prior to reweighing. If asbestos were cooled in the open room, moisture from the air would be absorbed and weights would differ.
When asbestos fibers are dehydrated, they change in such properties as mechanical strength. Water content in asbestos includes hygroscopic moisture adhering to the asbestos sur- face and the chemically combined water of crystallization.
The content of hygroscopic moisture in asbestos has no rela tionship to its chemical composition. It is directly related to such an environmental condition, as degree of relative humidity in the air. Removal of this moisture can be accom- pushed by subjecting asbestos to a temperature of approxi35 mately 212°F. At a relative humidity of approximately 40 per cent, moisture pickup in asbestos after equilibrium is reported to be approximately 1 per cent; at 70 per cent relative humidity, the total pickup is 1.5 per cent; at 95 per cent relative humidity, the total pickup is 2.5 per cent.
TABLE 2.10. EFFECT OF TEMPERATURE ON Loss IN WEIGHT
OF ASBESTOS FIBERS *
[click to enlarge]
*
Can. Mining and Met. Bull. (1951).
! Iron changing in weight caused by oxidation.
The Acid Resistance of Asbestos: Acid Washed Asbestos Fiber
The acid resistance of asbestos is important in a number of such different applications as corrosion resistant plastic, cement equipment, and filters. In some applications, it is desirable to remove all metallic and foreign matter. Mechanical devices as well as chemical, cleaning or washing processes are used.
With regard to the chemical process, the general proce- dure is to immerse asbestos in a boiling solution of 15 to 25 per cent hydrochloric acid. One to two hour immersion is generally sufficient. After the acid washing, the asbestos is subjected to water rinsing.
The water will remove all free chlorine and neutralize the fiber. This process generally produces a loss of approximately 10 per cent, by weight, of . the original asbestos. Loss in weight is due to removal of the metallic and foreign matter.
The acid washed fibers are dried in order to make them useful in such other processes as treatment with plastic or rubber resins.
...
Continue reading at ASBESTOS DEFINITION & COMPOSITION or select a topic from the closely-related articles below, or see the complete ARTICLE INDEX.
Or see these
Recommended Articles
- ASBESTOS, ITS INDUSTRIAL APPLICATIONS, ROSATO 1959
- ASBESTOS ORIGIN & NATURE - p. 1-36
- ASBESTOS PROPERTIES - p. 37-61
- ASBESTOS CEMENT PRODUCTS - p. 62-66
- CEMENT ASBESTOS PRODUCT MANUFACTURE - p. 66-73
- CEMENT ASBESTOS PRODUCT CURING - p. 73-75
- CEMENT ASBESTOS SHEET PRODUCTS - p. 75-78
- CEMENT ASBESTOS PIPE MANUFACTURE - 78-85
- ASBESTOS TILE MANUFACTURE - p. 86-93
- ASBESTOS INSULATION - 94-105
- ASBESTOS ELECTRICAL WIRE INSULATION - p. 106-112
- ASBESTOS FRICTION MATERIALS - p. 113-129
- ASBESTOS TEXTILES - p. 130-141
- ASBESTOS in PLASTICS - p. 142-177
- ASBESTOS PACKINGS & GASKETS - p. 178-184
- ASBESTOS FILTERS - p. 185-193
- ASBESTOS OTHER PRODUCTS - p. 194-200
- ASBESTOS BIBLIOGRAPHY - p. 201-207
Suggested citation for this web page
ASBESTOS CHEMICAL PROPERTIES at InspectApedia.com - online encyclopedia of building & environmental inspection, testing, diagnosis, repair, & problem prevention advice.
Or see this
INDEX to RELATED ARTICLES: ARTICLE INDEX to ASBESTOS HAZARDS
Or use the SEARCH BOX found below to Ask a Question or Search InspectApedia
Ask a Question or Search InspectApedia
Try the search box just below, or if you prefer, post a question or comment in the Comments box below and we will respond promptly.
Search the InspectApedia website
Note: appearance of your Comment below may be delayed: if your comment contains an image, photograph, web link, or text that looks to the software as if it might be a web link, your posting will appear after it has been approved by a moderator. Apologies for the delay.
Only one image can be added per comment but you can post as many comments, and therefore images, as you like.
You will not receive a notification when a response to your question has been posted.
Please bookmark this page to make it easy for you to check back for our response.
IF above you see "Comment Form is loading comments..." then COMMENT BOX - countable.ca / bawkbox.com IS NOT WORKING.
In any case you are welcome to send an email directly to us at InspectApedia.com at editor@inspectApedia.com
We'll reply to you directly. Please help us help you by noting, in your email, the URL of the InspectApedia page where you wanted to comment.
Citations & References
In addition to any citations in the article above, a full list is available on request.
- [1] ASBESTOS HISTORY & PROPERTIES [Book online] D.V. Roasato, engineering consultant, Newton MA, Reinhold Publishing Co., NY, 1959, Library of Congress Catalog No. 59-12535. We are in process of re-publishing this interesting text. Excerpts & adaptations are found in InspectApedia.com articles on asbestos history, production & visual identification in and on buildings.
- [7] Asbestos products and their history and use in various building materials such as asphalt and vinyl flooring includes discussion which draws on ASBESTOS, ITS INDUSTRIAL APPLICATIONS, ROSATO 1959, D.V. Rosato, engineering consultant, Newton, MA, Reinhold Publishing, 1959 Library of Congress Catalog Card No.: 59-12535 (out of print, text and images available at InspectAPedia.com).
- Other US EPA Publications on asbestos: web search 01/20/2011, see http://www.epa.gov/asbestos/pubs/pubs.html
- In addition to citations & references found in this article, see the research citations given at the end of the related articles found at our suggested
CONTINUE READING or RECOMMENDED ARTICLES.
- Carson, Dunlop & Associates Ltd., 120 Carlton Street Suite 407, Toronto ON M5A 4K2. Tel: (416) 964-9415 1-800-268-7070 Email: info@carsondunlop.com. Alan Carson is a past president of ASHI, the American Society of Home Inspectors.
Thanks to Alan Carson and Bob Dunlop, for permission for InspectAPedia to use text excerpts from The HOME REFERENCE BOOK - the Encyclopedia of Homes and to use illustrations from The ILLUSTRATED HOME .
Carson Dunlop Associates provides extensive home inspection education and report writing material. In gratitude we provide links to tsome Carson Dunlop Associates products and services.