Blue vs Yellow Flames & Efficient Oil or Gas Fuel Combustion
Blue vs Yellow Flames & Efficient Oil or Gas Fuel Combustion
- POST a QUESTION or COMMENT about Blue vs Yellow flames in heating equipment
Gas flame colors indicate proper or improper equipment operation.
Here, courtesy of aerospace engineer Herman Vogel, we discuss the relation of blue flame and efficient oil or gas combustion in engines or in heating equipment.
Mr. Vogel explains how the blue flame theory found its way into the later unsafe BlueRay Heating equipment line. Our page top photo shows a blue-colored flame photographed on a heating boiler burner using natural gas fuel.
InspectAPedia tolerates no conflicts of interest. We have no relationship with advertisers, products, or services discussed at this website.
Theory of Blue Gas Flames (Blueray) and What it Really Means to be Combustion Efficient
 Herman Vogel , Aerospace Engineer
Herman Vogel , Aerospace Engineer
I used to design combustors for Pratt&Whitney Aircraft (P&WA).
Later on it was top-secret government work for the InfraRed Countermeasure world where we created combustor-driven systems that would confuse heat seeking missiles from acquiring hot jet-engine targets.
This was back in the late 60's early 70's, a few years before the concept of BlueRay came out!
Why Blue Flames Indicate Superior Combustion Efficiency
Our combustors used JP [jet propulsion] fuel and always ran a blue flame (yellow flames existed only in-between re-setting for new thrust states requiring readjustments in fuel and air flows).
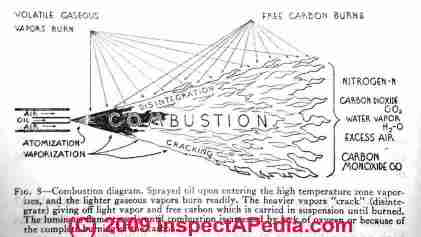
Our sketch shows how an oil burner gun atomizes and sprays heating oil into the combustion chamber - Audel Oil Burner Guide [free online textbook.]
Measure a blue flame temperature and you get around 3,000F. Measure a yellow flame temperature and you get about 1,400 to 2,000F at best (depending on how well the combustor atomizes liquid fuel and mixes it with oxygen). Just from temperature alone, one recognizes the superior aspects of blue flames.
Blue flames run closer to stoichiometric conditions of combustion, that is they burn as if using a pure gaseous fuel as opposed to liquid.
See COMPLETE COMBUSTION, STOICHIOMETRIC for details.
Gases basically burn 100% efficient or stoichiometrically. Jet engine manufacturers like P&WA, GE, and RR all take great pains in trying to design their combustors to first convert liquid fuel into fine atomized liquid droplets, then convert these droplets into pure vapor before they are allowed to mix with pressurized air (only oxygen part of air) for near complete combustion.
This yields about 95+% efficient combustion today. What happens here is that the fuel surface area, available for mixing with oxygen and burning more effectively, increases by factors of 1000 over that available with only atomized droplets of fuel.
Blue Flame Efficiency and the History of BlueRay Heating Boilers in the 1970's
The BlueRay folks got wind of these concepts back in the early 70's (perhaps even hiring some of the jet engine engineers?) to create their widely touted and highly efficient combustion process technology.
It's a shame that their product couldn't survive the rigors required by both the residential and commercial heating furnace customers. Unfortunately, it took a lot more tender loving care to keep the BlueRay flame "blue" than your typical yellow flamed combustors.
And as is pointed out
at BLUERAY Recall, poorly tuned BlueRay's had a tendency to burn sooty and rich, emitting Carbon, CO and NOx in their exhaust.
Two Measurements of Combustion Efficiency
 If I burn a pound of fuel, and get 3,000F flame temperature out of it as opposed to 2,000F, I'm getting more heat (efficiency) out of the same amount of fuel. In fact, true thermodynamic combustion efficiency of any combustor (jet engine, car, furnaces, etc.) can be measured in two ways, with both relying on the stoichiometric chemical reaction formula for complete combustion;
If I burn a pound of fuel, and get 3,000F flame temperature out of it as opposed to 2,000F, I'm getting more heat (efficiency) out of the same amount of fuel. In fact, true thermodynamic combustion efficiency of any combustor (jet engine, car, furnaces, etc.) can be measured in two ways, with both relying on the stoichiometric chemical reaction formula for complete combustion;
- Ideal heating value:
We theoretically calculate the released adiabatic ideal heating value (IHV), per unit fuel, assuming complete burning and compare it to its measured heating value (MHV) obtained from a furnace-gun and calorimetric system, the absolute combustion efficiency is then simply MHV/IHV - Ideal flame temperature:
We theoretically calculate the released adiabatic ideal flame temperature (IFT) value per unit fuel with complete burning and compare it to its measured flame temperature (MFT) value using a high temperature, platinum-rhodium thermocouple (Pt-Rh TC) held directly into the flame, the absolute combustion efficiency is then simply (MFT - Tin)/ (IFT - Tin).
Since a TC is simpler and easier to use than gathering data with a calorimetric setup, option#2 is recommended. For JP fuels: IHV = 18,950 BTU/Lbm, and IFT = 3,850F.
Therefore yellow flames have an absolute maximum burning efficiency of roughly (2,000 - 70) / (3,850 - 70) = 51%, while blue flames have an absolute maximum burning efficiency of (3,000 - 70) / (3,850 - 70) = 78%.
The roaring and abnormal gas burner flame shown in our photo is discussed in more detail
at GAS BURNER FLAME & NOISE DEFECTS
What is the True Efficiency Indicated by Yellow Flames? - How Oil Competes with Gas as a Heating Fuel
Now, if a yellow furnace system (with heat gun, combustion-chamber and exhaust-pipe) can at best only deliver about 51% efficient running, according to absolute thermodynamic principles, how do furnace companies and maintenance technicians claim efficiencies of 85% or even 90% or better?
They have collaborated, industry wide, to re-define oil-burning efficiency according to more favorable terms that can compete with the gas industry.
While there is nothing wrong with that, the basic problem lies in allowing them to conveniently forget that they should not compare their home-grown heating oil efficiency to "real" and absolute efficiency values as with burning methane or natural gas in furnaces or even JP-fuels in jet engines.
[Click to enlarge any image]
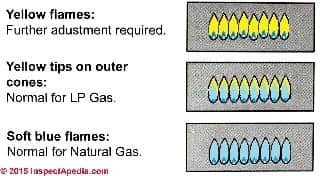
Our gas flame colour illustration shown at left, adapted from Bosch (2014),shows the properties of
- Unhealthy, mostly-yellow gas burner flames (top, check the gas regulator and air mix) that can occur on either LP (or propane) or natural gas burners
- Healthy yellow tips on outer cones of otherwise blue flames (center) characteristic of LP or propane gas burner flames
- Healthy soft blue flames of a natural gas burner
More details are
at GAS BURNER FLAME & NOISE DEFECTS
A Comparison of Oil Fuel vs Gas Fuel Efficiency in Heating Equipment
Consumers don't know what they are really getting when the oil industry compare apples to oranges.
When a fuel-oil furnace that is burning No#2 home heating oil is said to burn 100% efficient (based on non-stoichiometric reactions, defined by the furnace industry as standard, and using CO and CO2 exhaust product ratios to "relatively" redefine efficiency), their real furnace flame temperature would actually measure only about 2,000F at best.
Obviously, this is nowhere near 100% operation based on the above calculated theoretical analysis.
Now as their hypothetical flame decreases in temperature, the CO and CO2 fractions will change, and, depending upon the ratio of these changes, a new burner efficiency of under 100% gets quoted.
Again, these are not absolute thermodynamic values, rather they are relative burning efficiencies based on a reference flame temperature of 2,000F which was arbitrarily chosen to represent 100% combustion efficiency.
One may as well state that if your furnace has been tuned and reads 85% efficiency, as arbitrarily based on its emissions of CO and CO2 , it is really burning at [51% x 0.85 or] 43% efficiency in terms of "absolute thermodynamic efficiency".
Compare this to any gas furnace burning 100% in "absolute thermodynamic efficiency" and you are effectively throwing out almost half of your heating oil value.
How Oil Fuel is Competitive with Gas Fuel for Heating buildings
While this is shocking and true, remember that oil heat has as yet certain redeeming qualities.
It is still cheap enough to allow this disparity to happen and yet be competitive in heating your home because of its 40% greater heating value and 7% greater flame temperature (IHV=18,950 BTU/Lbm, IFT=3,850F) compared to natural gas (IHV=13,660 BTU/Lbm, IFT=3562F).
See NATURAL GAS & PROPANE COMBUSTION PRODUCTS
While BlueRay technology tried to take advantage of this disparity in efficiency, sadly they lost out to the need for frequent maintenance visits and to keep the unforgiving technology properly tuned or produce killing CO gases.
Bad Design and BlueRay™ - Design Products For What People are Likely to Actually Do
 OPINION-DF: Perhaps the BLUERAY Recall and carbon monoxide hazards had as a root cause, the mistake of designing an oil burner that required a high level of expertise and great care in following tuning instructions precisely.
OPINION-DF: Perhaps the BLUERAY Recall and carbon monoxide hazards had as a root cause, the mistake of designing an oil burner that required a high level of expertise and great care in following tuning instructions precisely.
For example, when adjusting the air-fuel mix by changing the air shutter opening on the oil burner, the direction of change, from more lean to more rich versus adjusting from the more rich to the more lean position could be enough to leave the oil burner adjusted to an unsafe position that would produce dangerous carbon monoxide. Here is what the company's service bulletin said:
When setting the unit for proper air mixture always start with the air band fully open, close it until proper CO2 reading is reached.
Close it further - if CO2 continues to climb you are on the "right side of the curve" and should then re-open the air band to proper CO2 reading. If it does not continue to climb you are on the "wrong side of the curve" in air-starved mode and are producing carbon monoxide (CO).
The subtlety of having to care about the direction from which one makes an adjustment to a common oil burner device, when either way the adjustment appeared to end at the same setting was perhaps too much to ask of traditional oil heat service technicians who were accustomed to more than sixty years of oil burners hat were wonderfully tolerant of rough handling.
Across a very wide range of discussions of construction problems and failures, we often return to this point. Good product design should provide for what people are likely to do (in installation, service, maintenance, use) rather than what the designer thinks they should do.
This article series answers most questions about central heating and water heating systems to aid in troubleshooting, inspection, diagnosis, and repairs.
Reader Comments, Questions & Answers About The Article Above
Below you will find questions and answers previously posted on this page at its page bottom reader comment box.
Reader Q&A - also see RECOMMENDED ARTICLES & FAQs
On 2020-04-19 - by (mod) - gas flame varies between strong & weak, thermostat not satisfied, fan shuts off (sometimes)
 Occam's razor argues that we ought to look for the simplest explanation and to avoid blaming multiple clues for a single problem.
Occam's razor argues that we ought to look for the simplest explanation and to avoid blaming multiple clues for a single problem.
But from your description I'm worried that there are two problems (sorry Occam, and yeah yeah I know I'm probably wrong):
1. when the burner is ON and the flame is normal size and color then the burner is working properly: but in this condition the fan shuts off before the house is warm THEN
IF the burner is continuing to run
THEN
Check the position of the dial on the fan limit control: if the dial has turned to show that the plenum temperature has dropped below the FAN ON temperature, then the control is doing what it's supposed-to: avoid blowing cold air on building occupants.
See the diagnostics at FURNACE FAN CYCLES DURING HEAT
2. When the burner is "on" but the flame is very weak or small (or goes out)
IF you have made sure that there are no clogged or wrong-sized orifices or air shutters (as we discussed earlier)
AND there is no lack of LPG fuel supply
THEN
I suspect an intermittent gas valve failure or clog in gas delivery, the gas regulator at the tank or at the heater, or possibly even a problem with the thermocouple that proves the gas flame at the heater.
See diagnostics at GAS REGULATORS & APPLIANCE / HEATER CONTROLS
See also THERMOCOUPLE REPAIR / REPLACEMENT - troubleshooting & replacement
Or if the flame actually goes out (this is less likely on your older furnace)
see FLUE GAS SPILL SWITCH TRIPPING & RESET
3. When the furnace gas burner flame is weak it's no surprise that the furnace can't heat the house to the thermostat set temperature.
Watch out: you have been fooling repeatedly with the settings on the fan limit controller. Doing this is inherently dangerous because of the worrisome fact that pressing on or manually turning or messing with the dial on the limit control too-easily bends the springs in the control.
The result is a subtly-damaged safety control that no longer responds properly or accurately to temperature as sensed in the plenum.
The risk is an overheated plenum that is cracked, leaking carbon monoxide and killing you and others in the building.
So be sure you have properly located, installed, and tested CO and smoke detectors in your home.
A summary of what the FAN LIMIT CONTROL is doing under different conditions may help clarify all this.
Take a look at the articles organized under
FAN LIMIT SWITCH - home -
I hesitate to say this but beyond finding a burner fuel and flame problem to fix, I suspect your fan limit control is fouled-up; I'd install a new one and leave its factory settings un-touched.
Thanks for the nice comments about InspectApedia.com - it's been a full time project for over more than fifteen years; we work hard on our information and articles to make them unbiased, researched, useful, but I recognize that the data is never complete nor perfect. So we're particularly grateful when a reader finds the website useful. Your questions and discussion help me make the information better for both of us.
On 2020-04-23 by PJ
PJ said:
Here is an update on my furnace problems that I have been working on:
I ran the fan without the burners on the manual override to confirm that all vents throughout the house had air flow. I adjust them so that there was enough air flow in all areas. I put the heat on several times during the day but could not get the burners to catch or if they caught, they did not stay on for more than 5 minutes at a time.
Yesterday morning, the burners came on with a nice strong flame and remained on but the fan kept shutting off before the house could get any warm air. I adjusted the fan limit switch to 70, 100, 200 and raised the heat anticipator on the thermostat.
The burners stayed lit and the fan stayed on but the temperature never went above 60F. I adjusted the fan limit switch to 100, 140, 200 and put the thermostat up to 80F.
Eventually the thermostat reached 70F but never went over. When it finally got to this level, the burners started to stay on even though the fan would go off for awhile.
Several hours later, I put the thermostat down to 70 and the burners shut down and then the fan when off. But within a couple minutes the burners and fan both came back on. I adjusted the switch to 90, 120, 200 and the burners are still staying lit constantly with the fan running a long time and then shutting off for a couple minutes.
When the temperature reached 72, I put the thermostat down to 74 and the burners went out and then the fan shut off. It stayed off for about 5 minutes and then burners came on.
I am still trying to figure out why the furnace would run so long and never climb above 60F for hours. The flame is now of good size and blue with yellow tips. The burners were going out a lot last week and not catching at times though the pilot light has always been strong.
This is exactly the issues I had for the first time in the Fall and I kept adjusting the fan switch limit, the heat anticipator and the amount of vents were open until one day they were just working well and they stayed that way until last Monday when I had the problem of the small flames, burners staying on, fan going on and off and temperature staying at 60.
Thank you again for all the information you specifically mentioned to me as well as for this wonderful site. I have used it for years and this is the first time I have been at a loss as to what is going on. Thank you so much for all the work you have put into this site.
On 2020-04-19 - by (mod) - Very tiny blue flames from burners of my Armstrong Ultra 80 propane furnace without any yellow. Burner shutting down.
 Usually when the blower turns off *before* the thermostat is satisfied
Usually when the blower turns off *before* the thermostat is satisfied
that means that the plenum was overheating and the fan limit control turned off the burner for safety - to avoid heat-cracking (making it dangerous) the heat exchanger.
We discuss the common causes of that problem at FURNACE FAN CYCLES DURING HEAT
That's odd if your flame is too small
Check for a blocked air filter or crimped, collapsed, disconnected duct or for a dirty squirrel cage blower fan.
See details at AIR FLOW TOO WEAK
I don't think your thermostat is the cause of the problem but you can certainly take a look at
HEAT ANTICIPATOR ADJUSTMENT to get the thermostat set up correctly
You can leave the blower fan in manual ON
but we need to dig more deeply to diagnose what's happening and to be sure your gas fired heating equipment is safe:
being warm won't matter if we're warm but dead.
On 2020-04-19 by PJ
It was working fine until last Tuesday when the blower fan started to shut off before temperature was reached. Furnace had been off the day before because of abnormally warm weather. 500 gallon tank has LPG at 58% and delivery was in mid-March.
I adjusted the air shutters and the flame is larger with some yellow tips. The burners stay lit constantly even when the blower shuts off but the temperature never goes beyond 60F. I put the fan limit settings to 70, 130, 200 as I had done in the Fall when I had the same issue. It hasn't changed the blower shutting off too quickly.
Would putting the blower fan on using the manual override on the fan limit switch be ill advised as i don't know why it is shutting off?
The only other thing I remember doing in the Fall was cleaning the inside of the thermostat which had quite a bit of dust and adjusting the heat anticipator. I removed any dust again but could this issue come from a problem with the thermostat? It is an old mercury thermostat.
Thank you for all the information you supplied on the last reply. I appreciate the time you have taken to help me out.
On 2020-04-19 - by (mod) -
Some causes of problems in LPG or propane fuel delivery or similar causes of weak gas flame
- Inadequate combustion air supply
- LPG supply adequacy - very low tank, nearly empty, pressure can drop below what the regulator is trying to delive
- Gas piping diameter, number elbows, valves, obstructions, run length: too-small diameter can restrict fuel flow
- Gas pressure primary regulator at the tank - set wrong or damaged
- Gas regulator at the appliance - set wrong, or improperly-converted between propane and LPG
- Gas orifices at the burner - dirty, clogged, wrong size + the possiblity of an improper jetting or regulator setting for LP vs natural gas
- Gas / air mixture shutter adjustment - inadquate combustion air
- Cleanliness of or obstructions in the entire fuel or combustion air supply system, anywhere
- Are you about out of LPG ? When the propane tank is about empty the gas pressure begins to fall.
Important: It may be diagnostic to note when this problem first appeared and what had or had not been done to the whole system just prior to that
Watch out: any condition that is causing weak or unreliable gas flame on a natural gas or LP (propane) heating appliance is dangerous, risking fatal cabon monoxide poisoning of the building occupants
Most important: assure that there are properly-located, working CO & Smoke detectors
Some diagnostic are at GAS BURNER FLAME & NOISE DEFECTS
On 2020-04-19 by Anonymous
Thank you for listing some possibilities. I do have carbon monoxide and smoke detectors installed in working condition. I have been the sole caretaker of this furnace for 20 years since most technicians just want to put in a new furnace.
By fuel supply problem, do you mean something at the tank or within the furnace itself?
I was wondering if there was a problem with the gas valve or build up in the jets to the burners.
Both issues I would refer to my local heating company who unfortunately are not open as to the Michigan shut-down that is in place until May 1st. I was hoping that adjusting the air shutter might increase the height of the flame and give it the yellow tip your diagram shows.
Just was not sure if more air would be what was lacking. I appreciate the information you have supplied.
On 2020-04-19 - by (mod) -
It does look like a pretty small flame.
I don't know what the problem is, nor all the possibilities. Some include a regulator or fuel supply problem or an air shutter adjustment problem.
Watch out. Unless you have special training you are Living Dangerously - trying to adjust or modify your own heating system.
The risk is producing fatal carbon monoxide.
At the very least be sure you have working carbon monoxide and smoke detectors properly located, installed, and tested.
I would expect the gas heat service technician to check the burner adjustment and regulator and fuel supply. It's also possible, if the heater was converted between LP and natural gas, that that was not done correctly.
On 2020-04-19 - by (mod) -
It does look like a pretty small flame.
I don't know what the problem is all the possibilities include regulator or fuel supply problem or an air shutter adjustment problem.
Watch out. Unless you have special training you AR Living Dangerously to adjust and modify your own heating system.
The risk is producing fatal carbon monoxide.
At the very least be sure you have working carbon monoxide and smoke detector is properly located, installed, and tested.
I would expect the gas heat service technician to check the burner adjustment and regulator and fuel supply. It's also possible if the heater was converted between LP and natural gas that that was not done correctly.
On 2020-04-19 by PJ
Not a great picture but it does show the size of the flames and the color accurately. I had a problem in the Fall with the furnace not heating above 60F. I cleaned everything and adjusted the fan limit switch to 70, 110, 200 and it ran well all Winter till this week.
On 2020-04-18 - by (mod) - Very tiny blue flames from burners of my Armstrong Ultra 80 propane furnace without any yellow.
I'm not sure, PJ as one person's very tiny might be someone else's "normal" flame size. But from your description it sounds as if you need an experienced gas heating service tech on site.
Perhaps you can attach a photo of the gas burner and its flame when the furnace is running and we can comment further.
On 2020-04-18 by PJ
Very tiny blue flames from burners of my Armstrong Ultra 80 propane furnace without any yellow.
Do I need to adjust to let it have more air?
Furnace won't raise temperature over 60 degrees because blower keeps shutting off even thought burners all stay lit.
Clean tubing and openings at pressure switch, checked fan limit switch (90, 130, 200), cleaned burners, cleaned flame sensor, cleaned blower fan, put in new filter, checked all wires for any rust, grit or disconnection.
On 2018-11-04 - by (mod) - swap out my vent-less propane fireplace for a blue flame vent-less heater ?
Thank you for your comment Thomas.
If I can make the article more useful for you and other readers I'll certainly be glad to do that.
About swapping out your gas fireplace, before changing the any hardware I would want to try to get a better diagnosis of why your flame is burning with so much yellow.
Unless you're telling me that the particular heater that you have or gas fireplace that you have is described as working correctly and normally with the flame you describe, we should look further because improper combustion, as you know , may be unsafe.
There could be a problem with the regulator or pressure, combustion Air Supply or something else.
Let's start at GAS FIREPLACES & GAS LOGS
On 2018-11-03 by Thomas Clark-
Thanks for this article ("Blue vs Yellow Flames & Efficient Oil or Gas Fuel Combustion").
I've been searching with both Bing and Google, and I finally found this link on Bing. I'm trying to decide if it would be worthwhile financially to swap out my vent-less propane fireplace ("fp"
) (I'm guessing 30% blue flame and 70% yellow flame) for a blue flame vent-less heater ("bf").
The trick in finding the article link was asking directly about blue flame vs yellow flame efficiency as opposed to asking about a propane fireplace vs propane blue flame heater.
...
Continue reading at COMPLETE COMBUSTION, STOICHIOMETRIC - explaining the complete combustion of fossil fuels and the details as well as the significance (to non-engineers) of Stoichichiometric Combustion, or select a topic from the closely-related articles below, or see the complete ARTICLE INDEX.
Or see these
Gas or Oil Burner Troubleshooting Articles
- BANGING HEATING SYSTEM NOISES
- BLUE vs YELLOW COMBUSTION FLAMES
- BLUERAY Recall
- FAN LIMIT SWITCH
- COMPLETE COMBUSTION, STOICHIOMETRIC
- GAS APPLIANCE CONVERT LP-to-NATURAL GAS
- GAS BURNER FLAME & NOISE DEFECTS
- GAS BURNER SOOT CAUSE & CURE
- GAS BURNER PILOT LIGHT PROCEDURE
- GAS BURNER POPPING NOISES
- GAS BURNER RUMBLING CHUGGING NOISES
- GAS FIRED WATER HEATERS
- GAS FIREPLACES & GAS LOGS
- GAS IGNITER DIAGNOSIS & REPAIR
- GAS PRESSURES LP vs NATURAL GAS
- GAS REGULATORS & APPLIANCE / HEATER CONTROLS
- GAS REGULATOR NOISES - buzzing, humming, and other noises at gas appliance regulators
- OIL BURNER NOISE DIAGNOSTIC INDEX
- OIL BURNER INSPECTION & REPAIR
- OIL BURNER NOISE SMOKE ODORS - home
- OIL BURNER PUFFBACK EXPLOSION
- WATER HEATER SCALE DE-LIMING PROCEDURE
Suggested citation for this web page
BLUE vs YELLOW COMBUSTION FLAMES at InspectApedia.com - online encyclopedia of building & environmental inspection, testing, diagnosis, repair, & problem prevention advice.
Or see this
INDEX to RELATED ARTICLES: ARTICLE INDEX to HEATING SYSTEMS
Or use the SEARCH BOX found below to Ask a Question or Search InspectApedia
Ask a Question or Search InspectApedia
Try the search box just below, or if you prefer, post a question or comment in the Comments box below and we will respond promptly.
Search the InspectApedia website
Note: appearance of your Comment below may be delayed: if your comment contains an image, photograph, web link, or text that looks to the software as if it might be a web link, your posting will appear after it has been approved by a moderator. Apologies for the delay.
Only one image can be added per comment but you can post as many comments, and therefore images, as you like.
You will not receive a notification when a response to your question has been posted.
Please bookmark this page to make it easy for you to check back for our response.
Our Comment Box is provided by Countable Web Productions countable.ca
Citations & References
In addition to any citations in the article above, a full list is available on request.
- Thanks to aerospace engineer Herman Vogel, August 2010, for providing this explanation of blue vs yellow fossil fuel combustion flames, what flame color means for combustion efficiency, and an insight into the history and failure of the Blueray oil burner product line. Also See COMPLETE COMBUSTION, STOICHIOMETRIC - discussing the complete combustion of fossil fuels and the details as well as the significance (to non-engineers) of Stoichiometric Combustion
- Audels Oil Burner Guide, Installation, Servicing, Repairing, Frank D. Graham, 1940's edition (obsolete). Updated versions of this guide are available in various editions, 1947, 1950, 1955, 1958, 1959, 1962, 1965, 1967, and at prices from around $3.00 to nearly $70.00 - useful for simple, clear, but not current, explanation of how heating equipment works. The original retail price was $1.00. Used copies are available
- Our recommended books about building & mechanical systems design, inspection, problem diagnosis, and repair, and about indoor environment and IAQ testing, diagnosis, and cleanup are at the InspectAPedia Bookstore. Also see our Book Reviews - InspectAPedia.
- Domestic and Commercial Oil Burners, Charles H. Burkhardt, McGraw Hill Book Company, New York 3rd Ed 1969.
- National Fuel Gas Code (Z223.1) $16.00 and National Fuel Gas Code Handbook (Z223.2) $47.00 American Gas Association (A.G.A.), 1515 Wilson Boulevard, Arlington, VA 22209 also available from National Fire Protection Association, Batterymarch Park, Quincy, MA 02269. Fundamentals of Gas Appliance Venting and Ventilation, 1985, American Gas Association Laboratories, Engineering Services Department. American Gas Association, 1515 Wilson Boulevard, Arlington, VA 22209. Catalog #XHO585. Reprinted 1989.
- The ABC's of Retention Head Oil Burners, National Association of Oil Heat Service Managers, TM 115, National Old Timers' Association of the Energy Industry, PO Box 168, Mineola, NY 11501. (Excellent tips on spotting problems on oil-fired heating equipment. Booklet.)
- In addition to citations & references found in this article, see the research citations given at the end of the related articles found at our suggested
CONTINUE READING or RECOMMENDED ARTICLES.
- Carson, Dunlop & Associates Ltd., 120 Carlton Street Suite 407, Toronto ON M5A 4K2. Tel: (416) 964-9415 1-800-268-7070 Email: info@carsondunlop.com. Alan Carson is a past president of ASHI, the American Society of Home Inspectors.
Thanks to Alan Carson and Bob Dunlop, for permission for InspectAPedia to use text excerpts from The HOME REFERENCE BOOK - the Encyclopedia of Homes and to use illustrations from The ILLUSTRATED HOME .
Carson Dunlop Associates provides extensive home inspection education and report writing material. In gratitude we provide links to tsome Carson Dunlop Associates products and services.