Mechanics & Forces of Freezing Water
Mechanics & Forces of Freezing Water
Effects of ice & freezing water on buildings & pipes + the physics of freezing water
- POST a QUESTION or COMMENT about the force of freezing water and ice expansion in or on buildings, plumbing systems, and in nature at rivers, lakes, ponds, streams
This article describes the expansive force of freezing water, or the force exerted by ice as it freezes and expands. The pressure exerted by freezing water depends on temperatures and other physical conditions, but it can be tremendous - enough to lift buildings, burst pipes & plumbing fixtures, and crush the hulls of ships trapped in ice.
What is the definition of the strength or force exerted by freezing water as it forms ice; how much expansion occurs as water freezes into ice & what are the effects on building plumbing systems; how can we predict where frozen pipes will burst?
InspectAPedia tolerates no conflicts of interest. We have no relationship with advertisers, products, or services discussed at this website.
- Daniel Friedman, Publisher/Editor/Author - See WHO ARE WE?
Freezing Mechanics - The Forces of Freezing Water
 Here we describe the typical effects of freezing water and ice on buildings and on or in building plumbing pipes & fixtures or in appliances such as water tanks or heating boilers.
Here we describe the typical effects of freezing water and ice on buildings and on or in building plumbing pipes & fixtures or in appliances such as water tanks or heating boilers.
We also discuss the physics of freezing water, explaining why it's the surface of water that freezes and why running water or water stirred by a bubbler freezes more-slowly or not at all.
Further below we will discuss freezing lakes, ponds and rivers, but first we'll take a look at freezing pipes.
Photo: this copper water pipe solder joint was simply pushed apart by the force of ice freezing in the pipe. Look closely and you can see that the original solder joint was not well-made. Below we show the more-usual way frozen pipes leak: by bursting.
[Click to enlarge any image]
Article Contents
- MECHANICS of FREEZING PIPES
- EXPANSIVE FORCE of ICE - FREEZING WATER
- PHYSICS of FREEZING or THAWING LAKES RIVERS STREAMS
- PHYSICS of FREEZING - RESERCH
- HOW DOCK & BOAT BUBBLERS WORK to PREVENT ICE
Temperature at which pipes freeze vs when pipes will break
As we elaborate below, while water begins to crystallize into ice at 0°C (or 32°F), its expansive forces generally do not cause water pipes to burst until temperatures further fall to around 20°F or until temperatures remain below freezing for some protracted time period.
As water temperatures drop, water actually can become supercooled by a few degrees before it begins to actually crystallize into ice. That's because the expanding ice crystals give up latent heat, warming the surrounding water back up to 0°C.
When water freezes its volume, in the form of ice, increases by about 9% under atmospheric pressure.
If the melting point (or freezing point) is lowered by large increases in pressure, the increase in volume on freezing is even greater (for example 16.8% at -20°C (-4F).
Factors Affecting the Temperature at Which Water Pipes Will Freeze
The actual point at which water inside of water pipes freezes solid in a building is determined by a combination of factors including the following:
- The absolute temperature to which water pipes are cooled.
Water expands when freezing. As temperatures continue to fall below the freeze point, increases in hydrogen bonding among water molecules causes the crystalline structure to open up, increasing the expansive force of the ice. (IAPWS 2013).
This explains why frozen water or heating pipes are more likely to actually burst at lower temperatures than at temperatures just below freezing.
In contrast, it should be noted that the high-pressure ices (ice III, ice V, ice VI and ice VII) all expand on melting to form liquid water (under high pressure).
It is the expansion in volume when going from liquid to solid, under ambient pressure, that causes much of the tissue damage in biological organisms on freezing.
In contrast, freezing under high pressure directly to the more dense ice VI may cause little structural damage. (Chaplin ret 2018). - The ambient temperature
at the water pipes is below freezing - The exposure conditions of the piping:
The actual temperature below freezing ( 0°C or 32°F) to which the pipes themselves are exposed, including effects of drafts, wind and air-leaks that blow freezing air over pipes in a building cavity or interior space.
Moving cold air will conduct more heat away from an exposed water pipe than still air. - The duration of exposure of the water pipes to freezing
temperature - longer duration of exposure to temperatures at or just below freezing increases the risk that the pipes will freeze before the next warming period.
The thermal mass of surrounding building materials that have been above-freezing will provide some residual heat that can prevent water pipes from freezing immediately when temperatures fall below freezing. - The thermal mass of the water pipes
including pipe material and diameter (and presumably volume of water). The actual burst point for freezing pipes has more variables including the pipe material, thickness, and even possibly its overall diameter and shape. - The water chemistry and the presence of solutes
play a small role in the freezing point of water: higher mineral content, for example, may lower the freeze point.
The freezing point of typical ocean water (salinity of 3.5%) is about -1.9°C. The presence of salt in water lowers its freezing point though once temperatures drop to or below about 15°F the effect of salt in water on its freeze-point is no longer significant. - The presence or absence of insulation on water pipes
For a short term temperature drop, insulation on water pipes will offer some freeze protection. For a long term temperature drop below freezing that protection is eventually lost.
For periods of prolonged cold broken by brief temperature increases above freezing, pipe insulation may also prevent heat gain in the piping. - Water pressure,
or the altitude above sea level has only an insignificant effect on the freeze point of water in building piping systems.
Increasing pressure normally promotes liquid freezing, shifting the melting point to higher temperatures. (Chaplin ret 2018).
Reducing pressure reduces the melting point of ice (or freezing point of water). Chaplin notes that 13.35 MPa (486 psi) gives a melting point of -1°C. So you can see that pressure changes in conventional building water piping systems will never have a significant impact on the freezing point of water in the pipes.
A reader, cited below, poses this rule-of-thumb: 1000 psi lowers melt point of water by about 0.5 °C (0.9 °F).
In general, 20°F (-6.67°C) is a practical number for the point at which water pipes are likely to freeze solid in a period during which temperatures dip below freezing overnight but don't remain there for days at a time.
Effect of Altitude, Atmospheric Pressure, or Water System Pressure on the Freezing Point of Water
- The water pressure within the water supply or heating system piping, tanks, pumps and controls has an insignificant effect on the freezing or melting point of water / ice.
Above atmospheric pressure or at higher altitude, the freezing point of water rises, but only by a very small amount. If decrease the atmospheric pressure from 1ATM way down to 0.006ATM the freezing point of water will increase from 0.00°C to 0.01°C
The melting temperature of ice falls very slightly at higher pressures.
At lower pressures the freezing point of water is very slightly lower.
Interestingly, when we decrease pressure (such as by moving to a higher altitude) the boiling point of water falls much faster than does its freezing temperature.
[Click to enlarge any image]
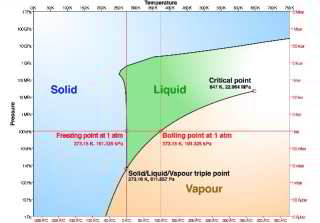
The phase change chart for water shown above, from Wikipedia commons, adapted from Chaplin, plots changes in the point at which water freezes as a function of pressure. You can see that at atmospheric pressure the freezing point of water is 273.2°Kelvin or 0°C or 32°F. Pressure is mapped on the vertical axes of the chart.
The nearly-perfectly-vertical line between the blue area (solid or frozen water) and green area (liquid water) forms of water mapped against the vertical axes giving pressure show that freezing point of water changes only very slightly in response to very large changes in pressure.
Does Leaving a Faucet Open Prevent Freezing Pipes from Breaking?
We know that running water, by moving warmer water from some building locations to colder pipe locations that would otherwise freeze, we can defer or even prevent frozen water supply pipes.
Watch out: at DE-WINTERIZE a BUILDING we warn that while running water may prevent supply pipes from freezing you may cause the building drain to freeze, block and even burst.
Some building experts advice that when faced with freezing pipes or already-frozen water pipes we open the faucets, reasoning that if pipes are frozen you might reduce the chances of freeze-burst piping or reduce its extent by opening faucets.
Allowing even a small amount of water (from the un-frozen pipe sections) to drain out of the building supply piping might reduce some of the in-pipe pressure even if no water is flowing from the faucet. - Building Research Council (1996)
Really? While there is no question about the tremendous force of freezing water it appears to be less clear the direction in which those forces work in plumbing systems. Let's look at the force exerted on building pipes along the length of the pipe vs. across the diameter of the pipe as ice freezes.
Horizontal freezing water-forces (ice pressure) along piping lengths
An exception to our horizontal split burst frozen pipe rule that describes what we have observed most often in buildings is the occasional separation of pipes at 90 degree elbow solder joint or even at a straight coupling or union (photo above).
In the photo above we show that clearly horizontal forces along the pipe pushed this pipe joint apart.
But look more closely at the solder joint and you will see that the original soldering job was poorly executed without proper cleaning and use of flux. The solder was not bonded to the copper and was not uniform in the joint - this was a weak point in the piping system so it's no surprise that the pipe failed here.
Lateral water freeze-forces (ice pressure) across building piping
 More often, where I observed that pipe joint separation a plumbing elbow (a common freeze point in buildings), often the elbow of bronze rather than just thin-walled copper resisted splitting while nearby softer copper piping did not.
More often, where I observed that pipe joint separation a plumbing elbow (a common freeze point in buildings), often the elbow of bronze rather than just thin-walled copper resisted splitting while nearby softer copper piping did not.
While expanding ice inside of water supply pipes may slightly increase pressure (as water is not compressible) our field experience and photos of frozen burst pipe such as the photographs at immediate left and again at below left suggest that most often frozen water pipes burst or split from the expanding ice pressure within a small section of pipe, creating first a bulge and then a split from forces across the diameter of the pipe rather than along its length.
It certainly appears from physical evidence that the water pipe shown in my photo bulged and then split by forces across the diameter of the pipe.
A rational view is that freezing water applies force in all directions rather uniformly. But the damage done by that force occurs where the force is confined.
But freezing water appears to be immediately confined more by the circumference of a pipe than along its length. Why might this be? Perhaps because the expansive force of ice increases as the temperature of the freeing water drops. A plug of ice forms in the pipe, the continues to expand as temperatures drop.
Why then might forces across the pipe be more confined than forces along the water pipe? We are not sure we not agree with one building expert who opines that the fill valve on toilets allows water, pushed along by freezing ice, to enter toilet tanks.
Really? Building water pressure varies over a range all the time for a variety of reasons (faucets open and shut, pumps starting and stopping) without ever pushing water through a closed toilet tank fill valve.
But there are building components that can absorb increasing water pressure:
- The water pressure tank
in buildings served by a private well or by a water pressure booster pump and tank system includes a compressible air reservoir - Cyclic water-consuming building fixtures
and appliances such as water heaters, boilers, steam boilers cyclically permit water to pass through water feed valves or pressure reducing valves into the appliance or fixture - Leaking, dripping faucets and running toilets
constantly relieve building water pressure (but sadly these conditions can cause a freeze blockage in a building drain)
What is the Expansive Force of Freezing Water?
 Expanding volume of ice:
Expanding volume of ice:
Our photo and note below illustrate the tremendous force exerted as water, freezing to solid ice, expands by about 9%.
Freezing force of water:
as the water-ice temperature continues to drop to 0°F the forces involved range between 25,000 psi and can continue to reach 114,000 psi.
Compare freezing water pressure with burst pressure resistance of copper pipes
Burst resistance of copper water pipes:
the working pressure of 1-inch L-copper piping is 420 psi, and the actual burst pressure is around 2650 psi.
Similarly a 1-inch type M annealed copper pipe has a working pressure of 286 psi and a bust pressure for drawn M-copper pipe of 3865 psi.
Smaller-diameter pipes have greater burst pressure resistance. For example, half-inch Type M annealed copper tubing has a working pressure of 420 psi and drawn copper type M has a burst pressure of 6135 psi.
You can see that the freezing force of ice is between 60 and 270 times greater than the working pressure of a 1-inch L-copper water pipe, and between 10 and 43 times greater than the actual burst pressure of the same copper piping.
At BANGING BOOMING NOISE DIAGNOSIS & CURE we cite the force and the loud noises produced by freezing, fracturing water-ice.
Our photo above shows thick slabs of ice from frozen Lake Superior, Two Harbors, Minnesota, in February 2019. The tremendous forces of freezing ice (expanding by 9%) causes fractures and fissures in the ice field that combine with wind and wave action to produce a cacophony of bangs, booms, creaks, and other noises as great slabs of heave into the jumble shown in our image.
How much does water expand or contract as a function of temperature and how much does ice expand? Various sources give different expansion forces for freezing ice.
Depending on its state, freezing water (or ice as temperatures continue to drop past freezing (32°F towards 0 °F) can expand by as much as nine percent at a maximum force between about 25,000 and 114,000 psi.
Actually water reaches its maximum density above freezing, at about 4°C. It is the expansion of ice as it continues to freeze that explains why icebergs float - the iceberg displaces a volume of water that weighs more than the (expanded) ice itself. The tip of an iceberg seen above water usually represents about 8% of the iceberg's total volume.
Stated another way we can explain why ice is lighter than water by explaining its expansion as water changes from liquid to frozen state.
As ice freezes forming hexagonal crystals (comprised of two H molecules join with an O molecule at an angle of 104°) the water in this form takes up more space than liquid water.
But the crystals formed by freezing water take on varying forms (and affecting the pressures exerted by confined ice) as temperatures continue to fall. - Debenedetti (2003)
Before modern physicists and engineers began calculating the expansive force of freezing water, Florentine academics had measured the strength of freezing water by enclosing water in a brass globe of known thickness and strength, then allowing it to freeze. Those academics observed that a one-inch globe of water, when freezing, could exert a 27,000 pound force. - Platts (1880)
 Luckily copper plumbing has some bending or flexing ability and does not break immediately. Copper water supply pipe strength varies by pipe type and wall thickness (K, L, or M copper) but break at around 3000 psi.
Luckily copper plumbing has some bending or flexing ability and does not break immediately. Copper water supply pipe strength varies by pipe type and wall thickness (K, L, or M copper) but break at around 3000 psi.
The thermal expansion of water is detailed
at THERMAL EXPANSION of HOT WATER.
Expanding ice forces are very strong when confined. Entire building movement caused by freezing and ice are discussed
at FOUNDATION DAMAGE by ICE LENSING
and at COLD WEATHER ROOF TROUBLE.
Watch out: while some experts advise leaving faucets open or dripping to avoid freezing pipes, this advice is risky if the building drains are exposed to freezing. In many areas the building main drain exits above the frost line and can be exposed to very cold conditions.
During normal plumbing use the surge of wastewater down the drain makes it past this cold spot without freezing. But a dripping faucet or running toilet, sending a small but continuous trickle of water down the drain can accumulate as ice until it expands, blocks the drain (leading to a sewage backup in the building) or until the drain line freezes and breaks.
On 2021-10-29 by inspectapedia.com.moderator (mod) - Build vs buy container capable of storing frozen water or keeping water from freezing by pressure?
 @Anonymous,
@Anonymous,
Thanks for a great question, that's an interesting idea.
The walls of any container, as they resist any force, including that of freezing water, in effect are exerting a "back pressure" - resistance = backpressure up to the point of bursting.
Shown above, an HDPE water storage container system than can withstand freezing, the Sagan Life Aquabrick(TM) provided by that California company.
From the article above on this page we have this key data:
When water freezes its volume, in the form of ice, increases by about 9% under atmospheric pressure.
If the melting point (or freezing point) is lowered by large increases in pressure, the increase in volume on freezing is even greater (for example 16.8% at -20°C (-4F).
It's true that the freeze-point of water increases slightly with pressure. But you need very large pressures to make any noticeable reduction in the freeze point of water.
What we didn't give in detail there were the effects on the freezing (or melting) point of water at very high pressures.
- To lower the freezing point of water from 32°F down to 24°F in a closed container you'd need to exert about 7,500 psia, and
- To lower the freezing point of water down to 16°F in a closed container you'd need about 15,000 psia.
- To lower the freezing point of water down to 0°F in a closed container you'd need to exert about 26,000 psia.
How to convert PSIG to PSIA
Remember that at sea level the true pressure or PSIA = 14.7 or 1 ATM (1 atmosphere). A typical water or tire pressure gauge that open to the atmosphere should be showing zero psig - it doesn't care that the atmosphere is pressing down on us at 14.7.
- PSIA = Pounds per Square Inch Absolute - pressure relative to zero or to an absolute vacuum.
- PSIG = Pounds per Square Inch Gauge Pressure - pressure read on a typical water tank gauge or tire pressure gauge, is actually measuring pressure above the pressure at sea level which is itself 14.7 PSIA.
- 0 PSIG = 14. PSIA
- 0 PSIA = an absolute vacuum or something more than minus or - 14.7 PSIG
- 1 PSIA = 1 PSIG + 1 ATM = 1 PSIG + 14.7 PSI
- 1 PSIG = 1 PSIA - 1 ATM = 1 PSIA - 14.7 PSI
You'd need a Really Strong Container to Resist Freezing Forces, or ... maybe we have a better idea
Perhaps we've missed something essential in your question that would explain why we'd want to try to engineer a new container to withstand freezing water by some extra-mechanical means and as well as to exert 15,000 or more psia of pressure as well as to somehow defrost or melt ice without an external power or heat source.
In my OPINION the design cost and product cost of an inventing, building, and producing automagic defrosting system that melted ice by back pressure exceeds, by many orders of magnitude, other solutions for storing water under freezing conditions.
The pressure of freezing water is enormous - enough to crush concrete.
Why not store your water in flexible containers that are not filled to the top. Many plastics can tolerate the amount of expansion from freezing water.
There are many such containers already sold, starting with ice cube trays open at the top, and moving on Silicone containers (your best bet in our OPINION), and thence to Nalgene, polycarbonates, and other plastics, styrofoam-lined water containers, and similar containers used in water, food and medical applications.
Even a simple freezer type "zip-lok" storage bag can, if not over-filled, safely store potable water that's frozen solid.

The cost of these containers is trivial compared with the highly-engineered and un-proven idea of a container strong enough to avoid bursting under the pressure of freezing water and to exert back pressure thought to re-melt the water - in essence providing its own mechanical anti-freeze container.
The science of the ability of these containers to avoid bursting as their water freezes is simple: the container contains sufficient free volume to accommodate the expanding water as it freezes, or it is sufficiently-elastic to do so.
- Help in these water freezing temperatures vs. pressures was found at the Engineering ToolBox, www.engineeringtoolbox.com "Ice / Water - Melting Points at Higher Pressure" where those smarties provide an online calculator to compute the melting point of water at various pressures.
We did not find an engineering analysis that argued that the back-pressure of a container also would melt the ice it contained. Rather, at sufficient pressure the freezing point of water is lowered.
On 2021-10-29 by Anonymous - design a water bottle that exerts back pressure on water when it starts freezing?
We can use insulating bottles to store water under subzero temperature. However, we can’t provide this insulation for a long time.
We know that the freezing point of water decreases with increase in pressure.
This is because water expands when it freezes. When water is freezing, it will expand and exert a pressure on the inside body of a bottle.
Can we device a water bottle which can exert a back pressure on water when it starts freezing?
The back pressure created can re-melt the ice and thus avoid any freezing?
Thus, explain what are the stresses a bottle experience when water transforms to ice?
The Physics Freezing or Thawing Ice
 Here we describe several factors that affect the formation of ice on large bodies of water like Lake Superior as well as on ponds, rivers, and streams.
Here we describe several factors that affect the formation of ice on large bodies of water like Lake Superior as well as on ponds, rivers, and streams.
Photo: in February 2019 the combination of very cold temperatures and low wind velocity led to thick solid freezing of the surface of Lake Superior such that we could walk offshore.
In the distance is L.C. who grew up on the North Shore of Lake Superior and whose comments about the behavior of ice on the big lake are included below in italics.
Above: currents play a role in the thickness and other properties of ice on Lake Superior as well as in smaller rivers and stream that drain into the lake; on the lake itself we often see an open water “river" next to thin or thicker ice. - L.C.
Factors affecting when ice forms on a lake, pond, stream & river
- Air temperature & water temperatures and temperature gradients.
Sunlight angle and duration are of course factors in both surface and air temperatures as well as other weather patterns.
Below-freezing air that cools surface water increases its density until the water reaches its maximum density at about 4°C, causing that colder water to sink.
That assures us that in freezing conditions over deep water there will be above-freezing water below the ice. - Agitation:
Wind and wind-created wave action, or other sources of water agitation,
illustrated below in this article in our photo of the ice chunks in moving water: agitation can lower the freezing point
Currents in rivers, streams and lakes, both horizontal currents and also vertical currents, such as up-currents caused by dock bubblers. Examples of these phenomena are shown in photos below.
Currents obviously will play a role in the thickness of ice on Lake Superior as we often see an open water “river" next to thin or thicker ice. - private email L.C. to DF 2021/11/29 - Weight:
of ice on water below.
Increasing weight will play a role in thickness, density, and then movement, most clearly seen in glaciers or flipping icebergs.
When I infamously (and perhaps foolishly) crossed Burlington bay [Two Harbors MN - Ed.] at night that teenage winter, I knew I was on very thick ice, maybe two feet thick with a thin layer of snow on top. But when I saw water under my footprints when I looked down, I panicked, thinking the ice might be breaking up and the wind might be pulling the ice on which I stood out of the bay.
I ran as fast I could toward the nearest point of shore, expecting to hit a lead and plunge into open water and have to swim for shore. - There was no lead, it was just the weight of that huge massive sheet causing the edge where it met the shore to buckle and water to seep up and then slowly seep out over the bay between the top of the ice and the layer of snow. - L.C.
- Friction
between moving surfaces on ice
Most obvious examples being sleds or skates or curling rocks, but most memorable for me was breaking huge sheets of ice from an ice ridge and balancing them on their edge then pushing them faster and faster over the smooth ice of the bay, releasing them to see how far they would “sail” before the wind would bring them crashing down and shattering into a thousand pieces! - L.C. - Water depth & volume
The sheer volume of water in Lake Superior means it takes a lot longer for it to freeze than surrounding bodies of water - often even after weeks of freezing temperatures, the bay won’t freeze until late January or February; and then the reverse as spring warms the land, we’ve seen ice chunks in the lake in June (though not for several years has it held on this long). - L.C.
 Our photo shows thick chunks of ice floating on the surface of Lake Superior in two Harbors Minnesota in February, 2018.
Our photo shows thick chunks of ice floating on the surface of Lake Superior in two Harbors Minnesota in February, 2018.
Air temperatures were well below freezing. So why wasn't the lake surface frozen solid?
We've said that currents that move deeper, warmer water up towards the lake or pond or river surface can prevent ice formation (freezing) in that area.
But it's not just the movement of warmer water up towards the surface that lowers the freezing point of water.
A second effect that lowers the freeze-point of water in a lake, pond, or stream is agitation, or stirring movement of the water itself.
Strong wind kicks up waves that so agitate the surface of the big lake that the solid freeze of the lake surface does not occur until either there are days of little or no wind, or temperatures are considerably below zero.
At the site where this photograph was taken we've seen open lake water when the still-air temperature was - 22°F (-30°C). But at those same very cold temperatures, when the wind drops the lake begins to freeze, as we show in our next photograph below.
When water at the surface is cooled to 4°C or 39.2°F it has reached its maximum density - a bit before it would freeze (which occurs at 0°C or 32°F).
This colder water begins to sink downwards through the warmer, less-dense water below, assuring us that deeper water is at a temperature above-freezing.
The fact that ice, less dense than water, floats also assures us that any ice will be at or near the water surface.
When water at the surface is cooled to 0°C and if the water is relatively calm so that there is not much active mixing of the cooled surface water layer with warmer water from below, the first ice forms as a thin layer.
Photo above: with the reduction in wind and waves the surface of Lake Superior begins to freeze, as shown in our photo of Burlington Bay, Two Harbors MN in February 2018.
Our next photo, shown below, illustrates the tremendous power of wind and waves on the big lake: huge chunks of ice are broken up and tossed shoreward by wind and piled near shore until eventually temperatures fall low-enough and wind becomes mild-enough that the whole mess freezes solid.
On the Arctic Ocean, much larger than those in our Lake Superior photo below, one might imagine “smooth sailing” or smooth snowmobiling or skiing over miles of Arctic ice. But massive ice ridges form to create the most difficult and time consuming obstacles for explorers. - LC
Watch out: if you are not familiar with safe procedures for walking on or exploring frozen lakes, stay off the lake surface. Failure to spot thin ice, a condition that can occur in some areas even when surrounding ice is several feet thick, means that you can suddenly fall through the ice into freezing water where death is usually quick.
In the winter of 1953, our neighbor Stevie Stevenson, ever the explorer, walked out onto the frozen surface of the University of Richmond lake where, at the lake's Western end and 100 yards from shore, he fell through the ice. He was "just testing the ice" according to the newspaper. Stevie was rescued by the InspectApedia editor's father.
Details are at SKEPTICAL BOY, 11, LEARNS ABOUT ICE on U. of R. LAKE [image file] as reported in the Richmond News Leader in the winter of 1953.
Continuing: With continued exposure to below-freezing air, the surface is further cooled and the thickness of the ice layer at water top increases.
As ice is less dense than water it floats or remains at the top surface.
At this point we can be pretty-much assured that deeper in the water we've got water that is at least 4°C warmer than at the surface.
Now if we mix, agitate, or stir-up the water near the surface, say from waves on Lake Superior, the added energy from stirring itself is very small, not enough to much-prevent freezing.
But the mixing of the warmer water from below with the cooling water at the surface will slow the freezing of water in a stream, or on a body of water in windy conditions, or on the surface of water that is being stirred up by running stream water, or by an air bubbler, dock-de-icer or boat bubbler like those described above.
So if we agitate a body of water whose surface is exposed to freezing temperature or below, that water won't begin to freeze until the entire thickness or body of agitated water has been cooled to 0°C or below.
Research on the Freezing Point of Water vs Burst Pressure of Building Piping Systems
- Amini, Behnam, and Saleh Sharif Tehrani. "Simultaneous effects of salted water and water flow on asphalt concrete pavement deterioration under freeze–thaw cycles." International Journal of Pavement Engineering 15, no. 5 (2014): 383-391.
Abstract
The presence of water flow on road surfaces may lead to early deterioration of bituminous pavements. The adverse impacts of various anti-freezing materials on road surface performance have drawn the attention of many researchers.
However, the simultaneous effects of salted water and water flow on the deterioration of road surfaces, particularly under freeze–thaw conditions, have not been adequately addressed.
This research aims to study the combined effects of water flow and anti-freezing materials, which are usually present in the vicinity of asphaltic pavements during freeze–thaw cycles, on asphalt concrete deterioration. Two sets of asphalt concrete samples were prepared and subjected to six exposure states.
The samples were also tested in an abrasion test apparatus and subjected to normal and frictional forces. Marshall strength loss and weight loss of the samples were measured and the results were analysed.
The results indicated that the combined effects of water flow and de-icers under freeze–thaw conditions intensified the deterioration of asphalt concrete.
- ASTM B42-20 Red Standard Specification For Seamless Copper Pipe, Standard Sizes
- ASTM B75-02
Standard Specification for Seamless Copper Tube
Abstract:
This specification establishes the requirements for seamless round, rectangular, and square copper tube suitable for general engineering applications.
The tube shall be manufactured by such hot- and cold-working processes as to produce a homogeneous, uniform wrought structure in the finished product. It shall be cold drawn to the finished size and wall thickness.
The requirements and size availability of tube in the tempers shall conform to the specified requirements. Tensile and yield strength test, Rockwell hardness test, expansion test, microscopical examination, hydrogen embrittlement, electromagnetic test, hydrostatic pressure test, pneumatic pressure test, electrical resistivity, and chemical composition shall be made to conform to specified requirements. - ASTM B88-20 Standard Specification For Seamless Copper Water Tube, available from ANSI at https://webstore.ansi.org/
Abstract
This specification covers seamless copper alloy water tubes for general plumbing and similar applications in fluid conveyance. These water tubes made from UNS C10200, C12000, and C12200 copper alloys are commonly used with solder, flared, or compression-type fittings.
The materials should be cold-drawn to size and the tubes finished by cold working and annealing to produce the required temper and surface finish. When tubes are furnished in coils, annealing is done after coiling, while those furnished in straight lengths should be in the drawn temper. The numerical values in this specification are not presented in inch-pound units, but rather, in metric or SI units only. - ASTM B315-19
Standard Specification for Seamless Copper Alloy Pipe and Tube
Abstract
This specification establishes the requirements for seamless, copper alloy pipe and tube in nominal pipe sizes, both regular and extra-strong, and seamless tube in straight lengths for general engineering purposes. The material of manufacture shall be a cast billet, bar, or tube. The product shall be produced by hot-working or cold-working operations, or both.
Unless otherwise specified, the product shall be finished by such cold working and annealing or heat treatment. Only tensile, yield, or elongation test results shall be a basis for rejection based upon mechanical properties. At least two replicate analyses for each element with a limiting value shall be conducted under chemical analysis. The test specimen shall be of the size and shape to permit testing with the available test equipment.
The surface of the specimen shall be sufficiently flat and smooth to permit the accurate determination of hardness and shall be sufficiently free of scale and foreign material to permit the accurate determination of hardness.
Care shall be taken to avoid changing the material's condition through either cold working or heating, or both. The product shall be free from defects, but blemishes of a nature that do not interfere with the intended application are acceptable. - Bing, Hui, Ping He, and Ying Zhang. "Cyclic freeze–thaw as a mechanism for water and salt migration in soil." Environmental Earth Sciences 74 (2015): 675-681.
- Chaplin, Martin, WATER PHASE DIAGRAM [PDF of web article], London South Bank University, retrieved 2018/01/20 original source: http://www1.lsbu.ac.uk/water/water_phase_diagram.html
- Chaplin, Martin, EXPLANATION of the DENSITY ANOMALIES of WATER (D1-D22) [PDF of web article] London South Bank University, retrieved 2018/01/20 original source: http://www1.lsbu.ac.uk/water/density_anomalies.html
- Engineering Toolbox: Copper Tubes Type K, L and M - Working Pressures vs. Size, retrieved 2022/02/09, original source: https://www.engineeringtoolbox.com/copper-tubes-dimensions-pressure-d_84.html
- Fountain, Henry & Jeremy white, "Warning From the Artic Deep", The New York Times, 18 December 2021 p. 1, A10-A11, retrieved 2021/12/18, original source: https://www.nytimes.com/interactive/2021/12/13/climate/antarctic-climate-change.html
This important article explains in lay terms the effects of global warming, Antarctic winds, Antarctic currents on the melting of the Antarctic ice sheets and the concomitant movement of glaciers towards the sea that in turn complicate our understanding of the role of the oceans in absorbing vs. releasing carbon dioxide from and into the atmosphere and the effects of these on global warming and coming increases in sea levels. The authors include helpful graphics explaining how wind-generated wave activity stirs deep water bringing warmer artic waters to the underside of ice sheets, resulting in thinning and melting of Antarctic ice.
Excerpt:
The ocean’s dominant feature, extending up to two miles deep and as much as 1,200 miles wide, is the Antarctic Circumpolar Current, by far the largest current in the world. It is the world’s climate engine, and it has kept the world from warming even more by drawing deep water from the Atlantic, Pacific and Indian oceans, much of which has been submerged for hundreds of years, and pulling it to the surface.
There, it exchanges heat and carbon dioxide with the atmosphere before being dispatched again on its eternal round trip.
Without this action, which scientists call upwelling, the world would be even hotter than it has become as a result of human-caused emissions of carbon dioxide and other heat-trapping gases.
... global warming is affecting the Antarctic current in complex ways, and these shifts could complicate the ability to fight climate change in the future. - IAPWS, WHY DOES WATER EXPAND WHEN IT FREEZES? WHY DOES LIQUID WATER HAVE A DENSITY MAXIMUM? [PDF of web article] (2013) Dr. R.B. Dooley, The International Association for the Properties of Water and Steam, Email: bdooley@iapws.org Retrieved 2018/01/20, original source: http://www.iapws.org/faq1/freeze.html
Website Excerpt:
IAPWS is an international non-profit association of national organizations concerned with the properties of water and steam, particularly thermophysical properties, cycle chemistry guidelines, and other aspects of high-temperature steam, water and aqueous mixtures relevant to thermal power cycles and other industrial and scientific applications. - John Platts, "The World's Encyclopedia of Wonders and Curiosities of Nature and Art, Science and Literature: Representing Anatomy, Physiology, Phrenology, Astronomy, Botany, Geology, Natural History, Ichthyology, Mythology, Ornithology, Meteorology, Mineralogy, Chemistry, Zoology, Entomology, Biography, Etc." Union publishing house, 1880 - Encyclopedias and dictionaries - 958 pages [available as a Google e-Book.]
Quoting:
Astonishingly Expansive Force of Freezing Water. — Although cold, in general, contracts most bodies, and heat expands them, yet there are some instances to the contrary, especially in the extreme cases or states of these qualities of bodies.
Thus, though iron, in common with other bodies, expands with heat; yet, when melted, it is always found to expand in cooling again. Thus also, though water expands gradually as it is heated, and contracts as it cools, yet in the act of freezing it suddenly expands again, and that with an enormous force, capable of rending rocks, or bursting the very thick shells of metal, &c.
A computation of the force of freezing water, has been made by the Florentine academicians, from the bursting of a very strong brass globe or shell by freezing water in it ; when, from the known thickness and tenacity of the metal, it was found that the expansive power of a spherule of water only one inch-in diameter, was sufficient to overcome a resistance of more than twenty-seven thousand pounds, or thirteen tons and a half. - Liu, Jianpeng, Ping Yang, and Zhaohui Joey Yang. WATER AND SALT MIGRATION MECHANISMS OF SATURATED CHLORIDE CLAY DURING FREEZE-THAW IN AN OPEN SYSTEM [PDF] Cold Regions Science and Technology 186 (2021): 103277.
Highlights
Propagation of the freezing front is highly dependent on salt content.
The matric suction gradient is the driving force of water-salt migration.
Water-salt migrations are more significant in specimens with lower salt content.
Water molecule mobility reveals water-salt migration mechanism.
Abstract
Water and salt migration mechanisms are of great significance for understanding the frost heave and thaw settlement of saline soils during freeze-thaw (F-T). In this study, unidirectional open-system F-T tests were conducted on saturated clay specimens with various chloride salt contents to investigate water and salt migration mechanisms.
Relevant parameters, including temperature, unfrozen water content, bulk electrical conductivity, matric suction, and water intake volume, were measured continuously during testing.
Besides, the water molecule mobility was obtained by the Nuclear Magnetic Resonance relaxometry tests to help understand the water and salt migration mechanisms. The test results indicated that the freezing front development was highly dependent on salt content.
The matric suction gradient was found to be the driving force of water-salt migration, which was larger in specimens with lower salt content and led to more water intake.
The migration of water and salt was asynchronous due to their different driving mechanisms during F-T. Specifically, salt migration was affected by both convection and diffusion, while water migration was primarily driven by convection.
This phenomenon was also confirmed by the different patterns in water and salt redistributions after F-T. Meanwhile, pore water in the frozen specimens with different salinity and the same unfrozen water content was found to have the same water molecule mobility.
Both the matric suction and water molecule mobility were dependent on unfrozen water content but independent on salt content.
However, the salt content has a significant effect on the soil freezing point depression, which further affects the matric suction and water molecule mobility. - Ma, Tiantian, Changfu Wei, Xiaolong Xia, Jiazuo Zhou, and Pan Chen. "Soil freezing and soil water retention characteristics: Connection and solute effects." Journal of Performance of Constructed Facilities 31, no. 1 (2017): D4015001.
Abstract excerpts
The relation between the soil-water characteristic curve (SWCC) and the soil freezing characteristic curve (SFCC) is investigated based on the similarity between the freezing/thawing and drying/wetting behaviors of soils.
...
Our experimental results show that at low water content the matric potential in the soil saturated with pore water and pore ice is generally different from that in the soil saturated with pore water and pore gas. A series of experiments on the soil samples saturated by NaCl solutions of different concentrations were performed to determine the osmotic potential and the water retention characteristics.
Based on the principles of surface chemistry, a relationship between the adsorptive forces and the water film thickness is developed, which is capable of describing the soil water characteristic when the sorption effect is significant.
Our results indicate that the freezing-point depression method should be applied with caution in determining the soil water retention characteristics of unsaturated soils at lower water content.
- NASA, "Can Sea Water Freeze" Aquarius Education & Public Outreach [from NASA) - retrieved 2023/05/10, original source: salinity.oceansciences.org/learn-more.htm?id=12
- "An Investigation into Freezing and Bursting Water Pipes in Residential Construction Laboratory and field tests conducted to discover the mechanics of freezing and bursting of pipes and why they freeze", 51 pages, 1996, Building Research Council, School of Architecture University of Illinois at Urbana-Champaign, Building Research Council Order Dept 1 East St. Mary's Road Champaign, Illinois 61820, Tel: 800-336-0616, Website: http://brc.arch.uiuc.edu Contact: Jeff Gordon, jrgordon@uiuc.edu
- "Properties of Water", retrieved 2018/01/20, original source: https://en.wikipedia.org/wiki/Properties_of_water
Wikipedia provided background information about some topics discussed at this website provided this citation is also found in the same article along with a " retrieved on" date. Because Wikipedia and other website entries can be amended in real time, we cite the retrieval date of such citations and we do not assert that the information found there is always authoritative.
A more-detailed chart of the freezing and boiling points of water as a function of pressure and temperature, also ascribed to Wikipedia but no longer available there, is provided by https://www.pilotsofamerica.com/community/threads/freezing-point-of-water.45415/ and is shown by clicking on our version of
this FREEZE POINT OF WATER [Illustration] used in the article above.
Another nice version of this chart is given by Chaplin, cited above
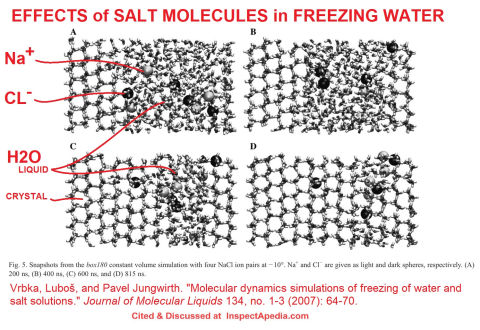
- Vrbka, Luboš, and Pavel Jungwirth. MOLECULAR DYNAMICS SIMULATIONS OF FREEZING OF WATER AND SALT SOLUTIONS [PDF] Journal of Molecular Liquids 134, no. 1-3 (2007): 64-70.
Abstract
Results of extensive molecular dynamics simulations of freezing of neat water and aqueous sodium chloride solutions are reported.
The process of water freezing in contact with an ice patch is analyzed at a molecular level and a robust simulation protocol within the employed force field is established.
Upon addition of a small amount of NaCl brine rejection from the freezing salt solution is observed and the anti-freeze effect of the added salt is described.
Excerpt from Summary and Conclusions:
The freezing and salt rejection processes are analyzed at a molecular detail in terms of the time evolution of the density profiles and hydrogen bonding patterns, and a robust simulation protocol within the
employed force field is established.
- Wolfe, Joe, "Freezing point depression and boiling point elevation: the effects of solutes and of pressure", [web article] UNSW School of Physics, Sydney Australia, (2010), retrieved 2018/01/19, original source: http://www.animations.physics.unsw.edu.au/jw/freezing-point-depression-boiling-point-elevation.htm
...
Reader Comments, Questions & Answers About The Article Above
Below you will find questions and answers previously posted on this page at its page bottom reader comment box.
Reader Q&A - also see RECOMMENDED ARTICLES & FAQs
On 2023-05-10 by InspectApedia Editor (mod) - How does the amount of salt in a water solution effect the force of expansion when freezing?
@John from Brisbane,
Thank you so much for a wonderful question.
Bottom line answer: there is little or no effect of salt on the freezing FORCE of water as it forms ice and thus expands in volume.
Details:
NASA Aquarius (an education arm of the US Space Program) has noted, in an education bit on freezing of sea water, that [Paraphrasing by InspectApedia] salt ions (NaCl) in water arrange themselves around the water molecules (H2O) thus interfering with the tendency of the water molecules to form into spaced-out crystals as ice at freezing temperatures.
The effect is that the freeze-point of water containing salt is lowered (which is why "road salt" can act as a de-icer). But at very cold temperatures starting around 15 degF, the temperature effects leading water molecules to arrange themselves into a hard crystalline form (ice) overcomes the interference effects of the salt molecules, and water freezes solid.
(That's why sea ice is concentrated at the much colder temperatures closer to the poles of the earth.)
Here's reasoning that gets us closer to your question of the effect that salt in water has on the freezing force (expansion) of water or ice:
As water freezes the salt molecules are in effect pushed aside - that is, the H2O molecules join together, squeezing out the NaCl molecules.
As a result, frozen "salt water" will have very few salt molecules in that structure. (Vrbka 2007)
Therefore we conclude that once water is actually freezing, salt that it contained previously, has little or no effect on the expansive force of the ice itself.
Some wonderful illustrations of the process of freezing water and the presence and then squeezing out of salt molecules are offered by Vrbka and Jungwirth, cited below and illustrated here with a snapshot excerpted from that article.
Where does the salt go when salted water freezes?
In lay terms, it's "pushed aside" or outside of the ice itself. We think that's why you may see concentrations of salt "crust" on surfaces or at the edges of where salt was used as a de-icer. See Bing- 2015 for more on this.
Sources:
Liu, Jianpeng, Ping Yang, and Zhaohui Joey Yang. "Water and salt migration mechanisms of saturated chloride clay during freeze-thaw in an open system." Cold Regions Science and Technology 186 (2021): 103277.
Vrbka, Luboš, and Pavel Jungwirth. "Molecular dynamics simulations of freezing of water and salt solutions." Journal of Molecular Liquids 134, no. 1-3 (2007): 64-70.
Abstract
Results of extensive molecular dynamics simulations of freezing of neat water and aqueous sodium chloride solutions are reported. The process of water freezing in contact with an ice patch is analyzed at a molecular level and a robust simulation protocol within the employed force field is established. Upon addition of a small amount of NaCl brine rejection from the freezing salt solution is observed and the anti-freeze effect of the added salt is described.
- Copy here at
https://inspectapedia.com/plumbing/Molecular-dynamics-simulations-freezing-water-salt-solutions-Vrbka.pdf
"Can Sea Water Freeze" Aquarius Education & Public Outreach [from NASA) - https://salinity.oceansciences.org/learn-more.htm?id=12
Also relevant to the article above on this page
Amini, Behnam, and Saleh Sharif Tehrani. "Simultaneous effects of salted water and water flow on asphalt concrete pavement deterioration under freeze–thaw cycles." International Journal of Pavement Engineering 15, no. 5 (2014): 383-391.
Bing, Hui, Ping He, and Ying Zhang. "Cyclic freeze–thaw as a mechanism for water and salt migration in soil." Environmental Earth Sciences 74 (2015): 675-681.
On 2023-05-10 by John from Brisbane
How does the amount of salt in a water solution effect the force of expansion when freezing?
On 2023-05-01 by InspectApedia Editor (mod) - What is the possibility of freezing the water in the cooler of a gas turbine engine inlet air cooler with zero flow of chilled water
@Roberto Guasch,
Wow what a cool question, no pun intended.
If there is no water flowing through a tube at 46 degF there is no freezing risk from the surrounding environment, so I infer that you're asking about a cooling or super cooling effect that occurs in gas turbine engines.
So with apology, I think there is a gas turbine engine engineering theory and question, and maybe a test question lurking here that's beyond our expertise. I do know that there are several different types of coolers and of cooling going on, and you and your (I'm suspecting) test question haven't specified which we're applying:
- fogging
- wet compression
- evaporative cooling
- vapor compression
- vapor absorption cooling
or a combination with thermal energy storage
As wikipedia explains,
Good luck on your exam.
On 2023-05-01 by Roberto Guasch
What is the possibility of freezing the water in the cooler of a gas turbine engine inlet air cooler with zero flow of chilled water through the tube at temperature of 46 degrees F and Relative humidity of 65 % cooler tube made of copper
On 2022-12-19 by Mark
@InspectApedia (Editor), Excellent comments. Thank you so much for broadening my perspective on this.
On 2022-12-03 by InspectApedia (Editor)
@Mark,
Continuing:
and from engineeringtoolbox dot com we have this nice summary
Specific volume is the inverse of density, or the ratio of the volume to the mass of a substance:
v = V/m = 1/ρ [1]
where
v= specific volume, units typically [cm3/g] or [ft3/lb]
V = volume, units typically [cm3] or [ft3]
m= mass, units typically [g] or [lb]
ρ= density, units typically [g/cm3] or [lb/ft3]
It's interesting that at 4 °C or 39.2 °F the density of water is greatest at above freezing (this is why ice floats)
FWIW here are two example data points:
at 35degF for water the specific volume is
1.00007 cm3/g
or
0.11983 US gallons (liquid) / lb
At 32 degF the specific volume of water changes to
1.00013 mg3/g
or
0.1184 US gallons (liquid) / lb
On 2022-12-03 by InspectApedia (Editor) - even a small expansion can cause damage if the expansion exerts tremendous force across a very small area
@Mark,
Thank you for the discussion. In fact, even a small expansion can cause damage if the expansion exerts tremendous force across a very small area. That's how we see concrete and masonry damage in tall buildings. However if the freezing is occurring in a plastic line that tolerates the increase in volume there could be no damage at all. So the surrounding materials are important.
The volume increase of about 9.05% when water has frozen to solid ice is consistent with the pressure increase.
It may be helpful to think of Boyle’s Law - though that law pertains specifically to gases not liquid:
P1V1 = P2V2 as we discuss in this article series, or in text:
At constant temperature, the volume of a fixed amount of a gas is inversely proportional to its pressure.
I would add that water freezing in a cylinder can do some pretty strange things. It doesn't necessarily expand uniformly in all directions but will in fact tend to extrude itself when it's in a cylinder such as a pipe or a downspout. I've seen water for example freeze and form a column of ice that pushed out the top of an open downspout or pipe.
On 2022-12-02 by Mark
@InspectApedia (Editor), Thanks so much for your response; I was amazed to find a place to be able to ask about a technical issue like this--and especially to get an answer. I totally get the very high pressures generated inside a closed container with freezing water.
It just seems to me like there would be an additional equational consideration in terms of the volume being frozen, but I guess not? We are talking about a total volume of about 2.4 ml in a 12 inch stretch of plastic tubing, or about 0.22 ml/in. With a 9% increase in expansion, as you say, at 17 degrees F, an expansion to 0.24ml/in. I'm not sure that would cause a failure. Maybe. Thanks...
On 2022-12-01 by InspectApedia (Editor) - different expansion forces for freezing ice
@Mark,
Various sources give different expansion forces for freezing ice.
Depending on its state, freezing water (or ice as temperatures continue to drop past freezing (32°F towards 0 °F) can expand by as much as nine percent at a maximum force between about 25,000 and 114,000 psi.
On 2022-12-01 by Mark
How much pressure does frozen water create in a one foot long, 1/4 inch plastic tube, ID 1/8 inch with a volume of about 0.15 cubic inches? This is in plastic tubing rated to -40 F with a pressure rating of 160 PSI that will supply an outdoor fishpond in a zone that has freezing temps of 17-20F at night and typically warms above freezing in the daytime.
It seems the volume is an important consideration, but I can't figure out the math. (edited changed photo format to JPEG -- markbieri@gmail.com)
On 2022-02-09 by Inspectapedia Com Moderator - How do you determine whether it is expanding ice or water pressure that causes pipe bursts?
@John King,
In freezing conditions such as those we describe in this article, burst pipes are caused by pressure from ice - which is a tremendous force when water freezes, as we describe above on this page.
In freezing conditions, burst pipes are not ever caused by water pressure itself. Building water pressure at normal delivery levels - say between 20 and 70 or even 80 psi are nowhere near the bursting pressure of plastic, steel, or copper piping.
For example, the working pressure of 1-inch L-copper piping is 420 psi, and the actual burst pressure is around 2650 psi.
Even the lower of these two numbers, the working pressure, is more than four times the water pressure delivered in any residential structure, none of which would be expected to even approach 100 psi.
From the article above,
As the water-ice temperature continues to drop to 0°F, the forces involved range between 25,000 psi and can continue to reach 114,000 psi.
As you can see that ice-force is enormously greater pressure than will ever be achieved by water pressure itself.
On 2022-02-09 by John King
Hello! Thank you for providing valuable, distinctive information!
Regarding your two above photos for lateral and horizontal pipe breaks.... as I understand, your writing says expanding ice caused breaks. Other webpages' information would suggest that pipe ice clogs created water pressure that caused these breaks. How did you determine whether it was expanding ice or water pressure? Was there a visual method?
On 2021-11-28 by Inspectapedia Com Moderator (mod) - Explain the change in water temperature caused by bubblers or water agitation: When will the Lake Freeze Solid?
 @Jake,
@Jake,
Thanks for the interesting comment/question on thawing ice using air (or as we will explain, any source of movement in water).
We don't have all the data we'd need to offer a technically-complete answer (with numeric data) to your question, but below is a general explanation of the thawing effect of air bubbles or air movement in freezing water.
Remember that lakes and rivers freeze from their surface downwards when air temperatures fall and remain below freezing.
The top inches or feet of water are cooled by freezing-air temperature and begin to form ice.
Ice, as you will read on the page above, expands or occupies more volume than the water that formed it, so it wants to float or remain on the top of water. And cooled water, down to 4C, sinks.
So when air temperatures are very cold and as a water surface begins to freeze, water temperatures below the ice level will be warmer than at the surface.
What about your air pumped bubbler pipe? How is that working to melt ice?
The photo shown above is excerpted from an Amazon advertisement for the Ice Eater P500, a 1/2 Horse Power Pond, Lake, Ocean and Dock De-Icer, produced by Bearon Aquatics.
[Click to enlarge any image]
- You are sending air down a pipe into water below a frozen surface.
The air is at a temperature above freezing, so it is bringing some heat into the frozen area. - You are agitating the water,
causing the movement of warmer water up towards the freezing or frozen surface. - Warmer water rising to the surface melts the ice.
If the volume and speed of warmer water moving to the surface is sufficient, that melts the surface ice and then prevents ice from forming.
Agitating water to send a warm water current upwards towards the surface by using air bubbles is an old, w established method to prevent freezing that has been in use for at least a century - we used to install bubblers to prevent water from freezing against and crushing the hulls of small boats on the Hudson river in New York.
Bubblers are widely used to prevent ice damage to docks and similar structures.
 In those installations the bubbler is keeping water around the boat's hull in motion - bringing up warmer water to the surface where dropping air temperatures remove so much heat from the water that left alone and still the water would freeze.
In those installations the bubbler is keeping water around the boat's hull in motion - bringing up warmer water to the surface where dropping air temperatures remove so much heat from the water that left alone and still the water would freeze.
It's a combination, then, of movement (running water freezes later than still water) and heat transfer (lower warmer water is being brought to the surface) that explains how a boat bubbler system works and that may explain what you observed.
To be clear, air isn't the only way to agitate water and thus to prevent ice formation or to melt ice such as around docks or boats.
The Kasco brand lake and pond deicer or "dock bubbler" shown below uses an electrically-driven propeller to move warmer bottom-water upwards towards the water surface.
Excerpting from the product-description
This dock deicer bubbler is specifically designed with a high efficiency motor and propeller with 1750 rpm that creates a directional flow of water.
This pushes warm bottom water to the surface and provides an ice-free area of protection around your boat, dock, pier, or other in-water property.
More about freeze-proofing a building including pipe freeze protection can be found at
WINTERIZE - HEAT ON PROCEDURE
On 2021-11-28 by Jake - Can you explain to me the change in water temperature caused by this air pressure?
I have a 36" steel pipe, vertically entering the water. one the surface the 36" pipe is capped and under the water ( Approximately 7feet below the surface) the end of the pipe is open.
I hooked a compressor up to the pipe, and began filling it with air, until bubbles started to form under the surface of the frozen surface.
So in my mind I have created enough pressure to force the water down far enough that the air started to come out the end of the pipe under the frozen surface.
Then the water started to melt at the surface until the entire surface around the pipe had thawed out, and in fact further down the river a patch or two started to melt as well.
Can you explain to me the change in water temperature caused by this air pressure, is there a table out there somewhere that will tell me all of this.
I understand the pressure pushing the water out, but I cannot mathematically explain the change in water temperature around or in the pipe?
Reader Question: How do I prevent the frost from pushing my drain pipe upwards
4/16/14 Anonymous said:
How do I prevent the frost from pushing my toilet drain pipe up through the floor and dislodging the toilet from the flange on the floor. This is at a cottage.
Reply:
Anon, you ask an important question, for which, with apology, I have to speculate a bit to try to answer.
Normally the toilet waste line doesn't push up through a floor.
To fix your frost-heaved waste piping we need to know why it's happening. I speculate that
- water is running beneath your cottage floor slab - which means we need to fix that cause outside by fixing roof drainage, surface drainage, subsurface drainage, foundation drainage - otherwise there is real risk of frost damage to the structure itself.
- the slab is probably not below the frost line - which is something one might have to live with but to do so we need to keep water out.
- We explain frost push or frost heaving
at FROST HEAVE / EXPANSIVE SOIL CRACKS in SLABS
and we expand on the topic
at VERTICAL MOVEMENT IN FOUNDATIONS
A "band aid" approach that I don't like is chopping out a section of flooring and packing foam insulation around the drain line in the fantasy that we can protect it from frost heaves. We need to either install construction components below the frost line or keep water out from below the foundation and slab.
- There could be a more subtle problem pushing up the waste pipe, such as ice lensing - a topic we explain
at FOUNDATION DAMAGE by ICE LENSING but that's not where I'd start.
Instead, start outside looking at where and how water is saturating the ground under your foundation and slab. While you're at it, and since this is a cottage that perhaps is not occupied and heated all year, also take a look
at WINTERIZE - HEAT OFF PROCEDURE
Reader Question: what's the actual temperature at which building pipes freeze?
Thank you for your helpful information about freezing water pipes!
But I fear that your reference to 20°F will mislead many of your readers. It seems to be generally said on the Internet that when outdoor temps get down to 20°F or below that freeze/break of indoor pipes becomes more likely. This is quite different from the actual temp of the indoor pipe itself, that may freeze/break
It takes over 1000 psi to lower the freezing point of water by one degree F! It seems inevitable that the vast majority of water pipes would freeze (and presumably break?) long before the real temp of the pipe and the water inside got down to 20°F, which would require a pressure of over 10,000 psi.
-k seeking facts and understanding in Boston
As we elaborate below, while water begins to crystallize into ice at 0°C (or 32°F), its expansive forces generally do not cause water pipes to burst until temperatures further fall to around 20°F.
The actual burst point for freezing pipes has more variables including the pipe material, thickness, and even possibly its overall diameter and shape. But 20°F is a good number for the freeze point of pipes.
pressure lowers freezing point of water a slight amount
* www1.lsbu.ac.uk/water/density_anomalies.html Explanation of the Density Anomalies of Water
"Pressure reduces ice's melting point (13.35 MPa gives a melting point of -1 °C)"
"In water, this line is backward sloping with slope 13.46 MPa ˣ K-1 at 0 °C, 101.325 kPa."
Many people want to know if the pressure in water pipes lowers the freezing point. Almost all Internet info including WP is not helpful: science phase diagrams in unfamiliar units that do not show appropriate detail, only that the melt temp does not vary much with pressure under common water pipe conditions.
It would be great to have a graph of temp in C and F (not K) vs pressure in PSI for 0-10,000 PSI for inclusion in WP articles. Until then, 13.35 MPa converts to 1936.25 psi (pounds per sq. inch). We could say something like:
* Pressure in water pipes reduces the freezing point slightly: a pressure of 1936 psi lowers the freezing point to -1 °C.
The graph in the good Martin Chaplin LSBU article appears to show that 100 MPa (14,500 psi) would lower the melt temp by about 10 °C.
Typical water pipe pressures are usually around 100 psi, which apparently would lower the freezing point by a small fraction of a degree. If the slope at zero is 13.46 MPa per degC, that works out to 0.051 °C (0.092 °F) for 100 psi.
Or maybe a more useful rule-of-thumb to remember would be 1000 psi lowers melt point by about 0.5 °C (0.9 °F).
It would be a relevant factoid to include in some articles, if we could be sure of the science/physics facts. - Anonymous by private email 2018/01/11
Reply:
Thank you for the comments. In our article we do mention that the impact of water pressure on freeze-point of water in pipes is very small - perhaps I should say "not really a factor" because of the numbers involved.
About the freeze point, as we both know that water freezes at 32F (at sea level, for fresh water), [actually water super-cools a bit before it freezes] we can pretty much figure that water in pipes at that point, too, depending on a range of factors we list in the article above.
The 20 degree figure is, I agree, a subjective ballpark number based on field experience for occupied homes during a temporary power outage. Certainly a home that is unattended and /or that remains un-heated for a protracted period is likely to have frozen pipes at somewhat higher temperatures.
A separate question is the hard freeze temperature and the relationship between that and burst copper pipes. I pose that colder temperatures increase the risk of bursting - but we need some science to support that claim.
I'll review the article to add our discussion, for which I'm grateful, and we can both look for more data.
On 2021-03-19 by (mod)
And take a look at the company's "Green Stripe" pipe described as
"The Professional Choice in 100 psi rated CSA grade cold potable water certified poly pipe. The most popular uses include well and lake water intakes, commercial and residential irrigation systems and lake and farm pond water distribution. "
On 2021-03-19 by (mod)
@KenB,
sorry to say I don't know for sure;
I would give the manufacturer of your pipe a call to ask their advice.
For example, if you are using Blue Stripe IPEX from IPEX Homerite Products, that pipe is rated to withstand 100 psi. Your starting pressure is probably very low, just a few psi, so your pipe might be fine. Give IPEX a call and ask them - and do let me know what you're told.
IPEX HomeRite Products
1425 North Service Road East, Unit 3
Oakville, ON L6H 1A7
Tel: 289-881-0120
Customer Service:
Toll free: 866-777-8023
Fax: 905-670-5295
info@ipexhomerite.com
sales@ipexhomerite.com
I can add that recently we lived in an off-grid cabin in the northern U.S. during temperatures of -24 degF for several weeks and though our PEX water tubing froze, it did not split; so at least in that small-diameter (1/2 " PEX) and under those conditions we were OK. I was worried that even if the PEX didn't split there might be brass fittings with water in the that might freeze and break, but fortunately our pipe routing was all PEX, no fittings, in the area of freezing.
For lakeside water supply systems that must be kept operational in winter I've participated in the installation of an air-pump system that in essence pressurised the piping with air sufficient to push water down below the ice level in the lake each time the pump was turned off.
For lakeside water supply systems like yours that are not used at all during winter, emptying the piping is probably the simplest, and least-costly insurance against freeze damage to the piping, fittings, and pump.
On 2021-03-19 by KenB
@danjoefriedman,
Yes. I it will freeze at the top for sure. I forgot to add that I would use blue, or blue stripe pipe which has a much higher rating than what most people use for cottage/pull it out and empty it in the fall water systems. I’m wondering if given the higher rated thicker wall blue rated pipe and it’s flexibility would it be able to withstand freezing without splitting for the 1-2 feet out of the ice with water in it, as I would put a T drain fitting just above the water line.
On 2021-03-18 by (mod)
@Ken B, HDPE piping can withstand some freezing; as Mark Cramer says ... it depends: on the lowest temperature and on the particular freezing forces.
At the lakeside water supply systems with which I'm familiar the water is pushed down the pipe to several feet below the lake surface so that only air is in the pipe in the sections exposed to freezing.
In your description I would expect your pipe to freeze.
See details at
WATER PIPE FREEZE PROTECTION https://inspectapedia.com/plumbing/Pipe_Freeze_Protection.php
On 2021-03-18 by Ken B
Will modern polyethylene 1” 160psi or 200psi well water pipe coming out of a lake and drained to a couple of feet above lake level with water from that point down to the pump well below the frozen ice be able to expand and withstand the expansion of the frozen ice and not burst in that upper section of the pipe. It’s a question I think many cottages have and there doesn’t seem to be any info out there on the internet.
water in it
On 2020-12-29 by (mod)
Joel
The purse you suggest is not feasible nor reliable. Water piping will not be dead level throughout it's runs.
Plastic pipe can be damaged by freezing as may be it's connectors, fittings, fixture, though it is more tolerant of freezing than metal pipes.
Here in northern Minnesota in recent temperatures well below freezing I've seen some PEX freeze solid then than without damage... yet. Harder plastics like CPVC are more vulnerable as may s be brass elbows, couplings, unions, etc. used in PEX systems.
On 2020-12-29 by Joel House
I have a bet with a friend. In a manufactured home CVPC piping system is it required to air purge the entire system so that there is less than 1/2 the diameter of the CVPC pipe to avoid ice expansion contributing to split pipes? If there is a belly in a pipe will the water freeze and burst the pipe or will it expand upwards on both sides of the belly?
On 2014-04-17 by (mod)
Anon, please see your question and my detailed reply in the bottom of the article above = there I could include links and added information.
Let me know if you have further questions.
Daniel
On 2014-04-16 by Anonymous
How do I prevent the frost from pushing my toilet drain pipe up through the floor and dislodging the toilet from the flange on the floor. This is at a cottage.
...
Continue reading at HEAT SOURCES to AVOID FROZEN PIPES or select a topic from the closely-related articles below, or see the complete ARTICLE INDEX.
Or see these
Recommended Articles
- BANGING BOOMING NOISE DIAGNOSIS & CURE
- HEAT SOURCES to AVOID FROZEN PIPES
- HOT WATER PRESSURE EXPANSION RATE
- PIPE FREEZE-BURST PATTERNS
- THERMAL EXPANSION of MATERIALS
- WATER PIPE FREEZE PROTECTION
- WINTERIZE - HEAT ON PROCEDURE
Suggested citation for this web page
FREEZING FORCE of ICE at InspectApedia.com - online encyclopedia of building & environmental inspection, testing, diagnosis, repair, & problem prevention advice.
Or see this
INDEX to RELATED ARTICLES: ARTICLE INDEX to BUILDING FREEZE PROTECTION
Or use the SEARCH BOX found below to Ask a Question or Search InspectApedia
Ask a Question or Search InspectApedia
Try the search box just below, or if you prefer, post a question or comment in the Comments box below and we will respond promptly.
Search the InspectApedia website
Note: appearance of your Comment below may be delayed: if your comment contains an image, photograph, web link, or text that looks to the software as if it might be a web link, your posting will appear after it has been approved by a moderator. Apologies for the delay.
Only one image can be added per comment but you can post as many comments, and therefore images, as you like.
You will not receive a notification when a response to your question has been posted.
Please bookmark this page to make it easy for you to check back for our response.
IF above you see "Comment Form is loading comments..." then COMMENT BOX - countable.ca / bawkbox.com IS NOT WORKING.
In any case you are welcome to send an email directly to us at InspectApedia.com at editor@inspectApedia.com
We'll reply to you directly. Please help us help you by noting, in your email, the URL of the InspectApedia page where you wanted to comment.
Citations & References
In addition to any citations in the article above, a full list is available on request.
- Our recommended books about building & mechanical systems design, inspection, problem diagnosis, and repair, and about indoor environment and IAQ testing, diagnosis, and cleanup are at the InspectAPedia Bookstore. Also see our Book Reviews - InspectAPedia.
- In addition to citations & references found in this article, see the research citations given at the end of the related articles found at our suggested
CONTINUE READING or RECOMMENDED ARTICLES.
- Carson, Dunlop & Associates Ltd., 120 Carlton Street Suite 407, Toronto ON M5A 4K2. Tel: (416) 964-9415 1-800-268-7070 Email: info@carsondunlop.com. Alan Carson is a past president of ASHI, the American Society of Home Inspectors.
Thanks to Alan Carson and Bob Dunlop, for permission for InspectAPedia to use text excerpts from The HOME REFERENCE BOOK - the Encyclopedia of Homes and to use illustrations from The ILLUSTRATED HOME .
Carson Dunlop Associates provides extensive home inspection education and report writing material. In gratitude we provide links to tsome Carson Dunlop Associates products and services.