Galvanic Scale & Galvanic Corrosion
Galvanic Scale & Galvanic Corrosion
- POST a QUESTION or COMMENT about galvanic scale and corrosion
Galvanic corrosion of metals:
This article defines galvanic corrosion and explains the galvanic scale, the effects of corrosion on metal roofing, and an explanation of the galvanic scale and causes of corrosion between dissimilar metals in any application.
We explain the Causes & rates of corrosion between dissimilar metals; tables of the galvanic scale for building materials; Catalog of corrosion & rust sources in building components.
Here we explain the galvanic scale, the effect of corrosion caused when certain metals are placed in contact, and we provide examples of galvanic corrosion hazards that occur in buildings metal roofing, building electrical components, building plumbing components, and at underground oil storage tanks and oil piping systems. Links at the end of this article provide further detail about rust and corrosion on nearly every building component where corrosion is a particular concern.
InspectAPedia tolerates no conflicts of interest. We have no relationship with advertisers, products, or services discussed at this website.
- Daniel Friedman, Publisher/Editor/Author - See WHO ARE WE?
The Galvanic Scale and Its Role in Corrosion of Metals
 This article includes excerpts or adaptations from Best Practices Guide to Residential Construction (Steve Bliss, J Wiley & Sons) , by Steven Bliss, courtesy of Wiley & Sons.
This article includes excerpts or adaptations from Best Practices Guide to Residential Construction (Steve Bliss, J Wiley & Sons) , by Steven Bliss, courtesy of Wiley & Sons.
Here we explain the galvanic scale, the effect of corrosion caused when certain metals are placed in contact, and we provide examples of galvanic corrosion hazards.
[Click to enlarge any image]
Article Contents
- GALVANIC SCALE & METAL CORROSION
- DEFINITION of GALVANIC CORROSION
- DEFINITION of GALVANIC SCALE - table of noble vs. less-noble metals
- CORROSIVITY of COMMON BUILDING MATERIALS
- CORROSION PREVENTION APPROACHES
- METAL ROOF CORROSION - galvanic and other corrosion warnings
- ELECTRICAL COMPONENT CORROSION- Corrosion Protection for Electrical Panels, Wiring, & Grounding
- PLUMBING COMPONENT CORROSION - Galvanized to Copper Pipe Connections - Use a Dielectric Fitting to Avoid Corrosion
- UNDERGROUND OIL TANK CORROSION - Steel Underground Storage Tanks and Corrosion
Definition of Galvanic Corrosion
Galvanic corrosion is the damage to or deterioration of metal components caused by an electrochemical reaction (metallic ion formation and migration) that occurs when two different metals are in contact with one another, principally when they are wet by or submerged in a liquid that acts as an electrolyte. Galvanic corrosion occurs around the point of contact between the two metals. Salts or other ingredients that increase the conductivity of the water or moisture increase the activity of galvanic corrosion.
A common example of corrosion is seen in plumbing systems where a copper water pipe is connected directly to an iron or steel water pipe.
Galvanic corrosion can occur at both metals through the action of an electrical current (galvanic current) that occurs at the anodic and at the cathodic member of the pair of metals. The anode member of the pair of metals gives up metal as ions of that metal move to and are deposited on the cathode member of the pair
The severity of galvanic corrosion that will occur where two metals are in contact depends on several variables including at least the following
- Extent of dissimilarity of the two metals that are in contact and the resulting difference in electrode potentials of the pair of metals
- Condition of the surface of each metal: presence or absence of a protective film including the film caused by the corrosion product itself. - (North 1970)
- Electrolyte properties including ionic species, pH, conductivity, temperature, volume and flow rate.
- Effects of the local environment such as moisture/drying cycles, exposure to sun, seasonal variations in temperature, humidity, moisture
- Physical, geometric factors such as the areas of each metal, their distance, contact points and position and possibly even orientation - (Jia 2006)
- Metallurgical properties of the metals including the alloy mix, heat treatment, mechanical disturbance
- Other factors such as microbiological contributors to galvanic corrosion and chemical reactions and reversible electrode potentials - (Borenstein 1994) (Zhang 2011)
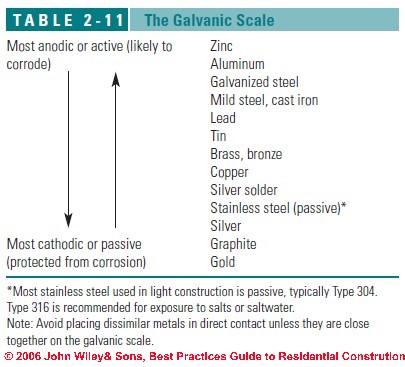
Definition of the Galvanic Scale: Tables of Noble to Less-Noble Metals
What is the Galvanic Scale
The galvanic scale (see Table 2-11) ranks a metal’s tendency to react in contact with another metal in the presence of an electrolyte, such as water or even moisture from the air
Some sources put graphite and platinum ahead of gold in resistance to corrosion while magnesium, zinc, and aluminum alloys tend to be among the most-easily corroded materials.
Metals at the top of the chart are called anodic, or active, and are prone to corrode; metals at the bottom are cathodic, or passive, and rarely corrode.
The farther apart two metals are on the chart, the greater their tendency to react and cause corrosion in the more active metal. Metals close to each other on the scale are usually safe to use together.
Relationship of Properties: Anodic vs Cathodic, Corrosion Prone vs Corrosion-Resistant, Least-Noble vs. Most-Noble, Highest Relative Voltage Potential vs Lowest Voltage Potential
All of these term pairs can be used as descriptors of the corrosion resistance of a metal.
Metallic Corrosion Scale Terms |
||
| Highest Resistance to Corrosion | Lowest Resistance to Corrosion | Comments |
| Cathode or Most-Cathodic | Anode or Most-Anodic | |
| More-Noble or Most-Noble | Less-Noble or Least-Noble | |
| Low or zero relative potential voltage | Higher relative potential voltage | Actually higher relative potential voltage will be a more negative number where the entire relative potential voltage scale ranges from zero volts (most cathodic) to minus or - 1.65 volts (most anodic) |
| Most Passive or Least Active | Least Passive or Most Active | |
| Graphite, Gold, Platinum, Titanium, Nickel, Alloy 20 Stainless Steel, Silver | Magnesium, Zinc, Beryllium, Aluminum Alloys, Cadmium, Mild Steel, Cast Iron | Copper and lead are closer to the middle of this scale; |
Notes to the Table Above
Metals that have relatively lower resistance to corrosion, when placed touching another metal with higher resistance to galvanic corrosion, will act as the anode and will suffer corrosion.
Example: copper pipe joined directly to mild steel or cast iron pipe will produce corrosion appearing more noticeably on the mild steel or cast iron pipe.
A detailed galvanic corrosion chart ranks various metals plotted against a scale that extends from most-noble or most-cathodic (most resistant to corrosion) metals to least-noble or least-cathodic (least resistant to corrosion).
The galvanic scale can also be expressed in voltage potential of these metals in an electrolytic solution (such as salty water), where the most-noble, most-cathodic, most-corrosion resistant metals have the lowest relative potential voltage (close to zero) while the least-noble, least-cathodic, least-corrosion resistant metals have the highest voltage potential.
"Least-cathodic" is the same as saying "most-anodic" when describing a metal that is most easily corroded.
Galvanic Scale Ordering for Metals Commonly Found in Buildings or Building Mechanical Systems
The metals in these lists are ordered from #1 - least noble, most anodic, most corrosion prone, to higher numbers = more noble, more cathodic, less corrosion prone. Some sources make slight re-orderings of pairs of metals in the lists given below, most likely to account for variations in alloys of metals in specific applications.
Other significant variations include the environment. For example metals on boats in salt water will show a more severe range of corrosion properties than in fresh water or above water.
Plumbing Metals: least-noble to most-noble
- Galvanized iron pipe = zinc plated mild steel pipes
- Mild steel pipes
- Cast iron pipe
- Lead pipe
- Yellow or red brass pipes
- Copper pipes
- Red bronze ( connectors)
- 50/50 lead-tin solder mix
- Stainless steel pipes & tubing grades 304, 316, 410, 416 or 430
Electrical Wiring & Connector Metals: least-noble to most-noble
- Aluminum or aluminum alloy wire or connectors
- Brass connectors
- Copper wire or copper connectors, also possibly copper-plated wires or connectors depending on effects of cuts, scratches, mechanical damage
- 90/10 copper nickel alloy
- 80/20 copper nickel alloy
- Silver (solder)
- Gold (or gold plated wires and connectors)
Roofing & Architectural Metals: roof surfaces or flashings: least-noble to most-noble
- Zinc roof flashings
- Aluminum sheet metal roofing or roof flashings
- Galvanized steel roofing (zinc-plated steel)
- Mild steel roofing (common corrugated metal roofing)
- Cast iron or wrought-iron components (railings, facades)
- Aluminum-bronze alloys
- Naval brass, yellow brass, red brass
- Copper roof or roof flashings
- Tin or tin-plated metal roofing or lead-tin solder (some metal roofs particularly antique)
- Stainless steel roofing (grade 410, 416)
- Lead roof flashings or lead-coated roofing (particularly antique)
- Stainless steel roofing (alloy grade 302, 304, 321, 347)
- Stainless steel roofing (alloy grade 316, 317)
- Stainless steel roofing (alloy grade 20)
Zinc-protected metals: Galvanized Metals: least-protected to most-protected against corrosion by zinc plating
When metals are protected by a zinc coating such as in some roofing applications, (galvanized metals) the galvanic scale ordering changes somewhat from the lists above.
- Pure zinc
- Aluminum with zinc protection
- Galvanized steel with zinc protection
- Cadmium with zinc protection
- Mild steel & wrought iron with zinc protection
- Cast iron with zinc protection
- Lead-tin solder with zinc protection
- Lead with zinc protection
- Brass or bronze with zinc protection
- Copper with zinc protection
- Stainless Steel with zinc protection
- Source: "Prevention of Galvanic Corrosion in the Roofing Industry", Shadowcrest Roofing, 265 Pawnee st. Unit C, San Marcos CA 92078, USA TEl: 760-593-0300, retrieved 2015/11/04, original source http://shadowcrestroofing.com/prevention/prevention-of-galvanic-corrosion-in-the-roofing-industry/
Examples of Measures to Control or Prevent Galvanic Corrosion on Building Components
In buildings and on building components or mechanical systems it is sometimes possible to prevent or retard harmful galvanic corrosion of metal components.
The following concepts can guide steps to minimize galvanic corrosion on or in buildings:
- Choose metals close together in the galvanic scale: If you must joint different metals (as opposed to using only one kind of metal) when combining the two metals (such as connecting two types of metal piping), pairing metals that are as close together as possible in the galvanic series will minimize galvanic corrosion.
- Prevent electrical connections: if two dissimilar metals are to be joined they can be separated by a non-conductive component such as a dielectric fitting on pipes
- Use corrosion-resistant connectors: when you must join two dissimilar metals such as copper to iron pipe, a brazed or soldered joint will be more durable than a mechanical or threaded joint
- Choose proper relative area or size of joined metals: when you must joint two dissimilar metals, if the less-noble or more anodic metal area is small compared to the higher-noble cathodic metal the corrosion rate will be greater
- Use protective coatings correctly: if using an anti-corrosive coating or paint be sure both metals are coated, don't just coat one of them
- Use a sacrificial anode where feasible - source: Shadowcrest Roofing, Op. Cit.
Measures to Protect Specific Building Materials from Galvanic Corrosion
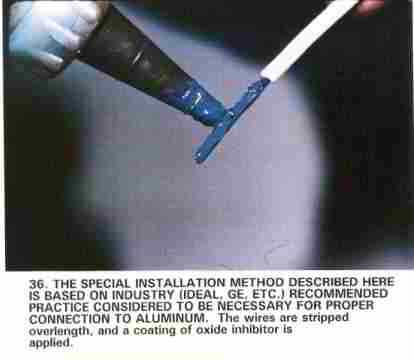
- Corrosion is controlled if not prevented at electrical connections, such as between wires or between a wire and a connecting lug by the use of an antioxidant paste (photo above). The antioxidant paste itself typically contains a less noble metal than the wire or connector and thus that acts as a sacrificial anode. A less noble metal is one that has less resistance to corrosion or chemical action than the metal to which it is being compared. Examples of high-noble metals that are very corrosion resistant include gold, platinum and silver.
See ALUMINUM WIRING REPAIR SPECIAL CONCERNS - An example of controlled galvanic corrosion is the use of a sacrificial anode in a water heater tank. The galvanic corrosion of the sacrificial anode in the water tank slows corrosion of the water tank body itself.
See ANODES & DIP TUBES on WATER HEATERS - An example of prevention of galvanic corrosion is the use of dielectric fittings, basically fittings that include either an intermediate metal (brass) or an insulating plastic component between connections of copper to steel water pipes.
See DIAELECTRIC PIPE FITTINGS - Metal roof corrosion is retarded or prevented by application of special coatings or by choice of a metal such as copper or special steel alloys that form their own protective oxide coating.
See TERNE METAL ROOFING
Galvanic & Other Corrosion Warnings for Metal Roofs
Corrosion Standards for Metal Roofs
Steel roofing materials are tested for corrosion-resistance in a salt spray cabinet per ASTM B117 and also in a condensation chamber per ASTM D4585.
Adapted/paraphrased with permission from Best Practices Guide to Residential Construction (Steve Bliss, J Wiley & Sons) , chapter on BEST ROOFING PRACTICES:
With metal roofing or any metal building components, the safest strategy is not to mix metals that come in direct contact with one another.
Use aluminum flashing and fasteners with aluminum roofing, copper flashing and copper nails with copper roofing, etc. When this is not possible, choose a second metal that is not likely to lead to galvanic corrosion or use a physical barrier to separate the two metals.
The Area Effect Determines the Rate of Metal Corrosion
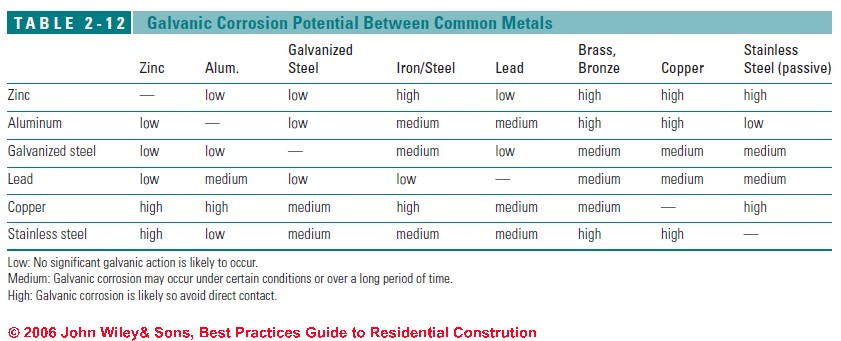
The rate of corrosion is controlled by the area of the more passive metal. For example, a galvanized steel nail (active) will corrode quickly if surrounded by a large area of copper flashing (passive).
If a copper nail is used in galvanized steel flashing, however, the corrosion of the steel will be slow and spread over a large area, so it may not be noticeable. In each case, the active metal corrodes, and the passive metal is protected.
Galvanic Corrosion of Metal Roofing
Because they are made from active metals, aluminum and zinc roofing panels, as well as steel roofing with aluminum and zinc coatings (galvanized steel, Galvalume®, etc.), are vulnerable to galvanic corrosion if allowed to come in contact with more passive metals. [Click any image or drawing to see a larger copy]
For example, never use copper or lead flashings with aluminum, zinc, or galvanized roofing materials. Even water dripping from a copper pipe, flashing, or gutter can lead to corrosion of coated-steel or aluminum roofing materials.
How common flashing materials react with metal roofing and other metal building materials is shown in Table 2-12 above.
Where incompatible metals must be used in close proximity, use the following precautions:
- Separate the two dissimilar metals with building paper, bituminous membrane, durable tapes, or sealants so they are not in direct contact.
- Coat the cathodic (less active) metal with a nonconductive paint or bituminous coating.
- Avoid runoff from a cathodic metal (e.g., copper gutters) onto an anodic metals (such as galvanized steel).
Other Incompatible Materials Found on Metal Roofs
In addition to galvanic corrosion, a number of other common building materials can harm the finishes on metal roofing or lead to etching or corrosion of the material itself:
Wet Mortar Effects on Metal Roofing
Aluminum roofing materials and aluminum based coatings can be damaged by alkali solutions such as wet mortar. Where contact with wet mortar cannot be avoided, one option is to spray the metal with lacquer or a clear acrylic coating to protect it until the mortar is dry.
Pressure-Treated Wood Effects on Metal Roofing
Roof panels treated with aluminum and zinc coatings should not come into direct contact with pressure-treated (PT) wood, which can damage the finish and accelerate corrosion.
Sealants & Caulks Impact on Metal Roofs
Use only sealants recommended by the manufacturer. Never use acid-cure silicones (the most common type, with a vinegar smell) or asphalt roofing cement with coated-steel roofing, as these will mar the finish. Commonly recommended products include butyl tape and gunnable terpolymer butyl or urethane sealant.
Salt Spray Impact on Metal Roofs
Saltwater spray is very hard on metallic coated– steel products and may lead to corrosion within 5 to 7 years. In these areas, the best choices are copper, stainless steel, or painted aluminum. Hylar/Kynar® finishes hold up best.
Also see our metal roofing home page,
METAL ROOFING
and
see CORRUGATED ROOFING
and
see COPPER ROOFING
-- Adapted with permission from Best Practices Guide to Residential Construction (Steve Bliss, J Wiley & Sons) .
Other Examples of Corrosion Between Dissimilar Metals and the Need for Dielectric Fittings in buildings
Corrosion Protection for Electrical Panels, Wiring, & Grounding
Corroded copper grounding wires can also be unreliable as our photo shows. The copper wire was bonded to a galvanized-iron water pipe where corrosion was exacerbated by the combination of dissimilar metals and wet conditions.
As we discuss at ELECTRICAL GROUND SYSTEM INSPECTION, we would be reluctant to trust this connection for the building grounding system.
You might also take a look at WATER PIPING GROUND BOND.
at ELECTRICAL GROUND PIPE CORROSION we describe how stray voltage into the ground system can cause plumbing leaks or even damage to air conditioners and heat pumps.
Also see ALUMINUM WIRING HAZARDS where corrosion may be a factor in the reliability of some aluminum wiring connections, particularly in damp or wet locations and where aluminum is joined to copper without using the appropriate connectors and antioxidants.
Corrosion in electrical components, possibly including galvanic effects can cause more subtle hazards such as poor connections inside of electrical panels, switches, and junction boxes.
"Phase II Report, Evaluation of Residential Molded Case Circuit Breakers", Wright-Malta Corp., (by J. Aronstein, for U.S. Consumer product Safety Commission, Project #CPSC-C-81-1455), March 10, 1984 (Contains experimental analysis of materials, construction, and performance of molded case circuit breakers, including FPE.
Lack of corrosion resistance of certain internal parts is considered to be a factor in the failure of the circuit breakers.
At above-left we illustrate an uninsulated aluminum grounding conductor that corroded through where it contacted a masonry block wall.
Galvanized to Copper Pipe Connections - Use a Dielectric Fitting to Avoid Corrosion
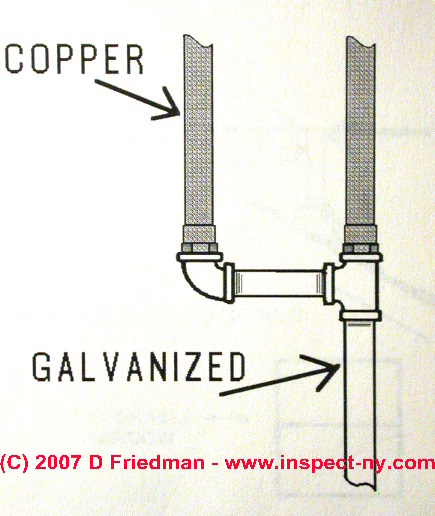
When connecting iron or galvanized iron pipes to copper in buildings, often corrosion and leaks will occur at the meeting of these two dissimilar metals.
Using a brass fitting to connect these two metals, or more commonly, using plastic or bronze fittings at the joint between these two metals will avoid future corrosion and leaks.
The photo (left) shows a galvanized iron union used to connect copper to galvanized iron. In the upper image you can just make out the black bronze ring built into this plumbing connector to avoid corrosion where the copper presses against the galvanized iron.
How do we explain that in some buildings we see direct copper-to-iron pipe connections with no corrosion? Luck? Maybe. But the corrosivity of the water is probably a factor in how rapidly copper-to-galvanized pipe connections will corrode and leak.
Spelling note that may help some searches: it's not dialectic pipe fittings, but dielectric pipe fittings.
Steel Underground Storage Tanks, Oil Piping, and Galvanic Corrosion
Dielectric material means a material that does not conduct direct electrical current. Dielectric coatings are used to electrically isolate UST systems from the surrounding soils. Dielectric bushings are used to electrically isolate portions of the UST system (e.g., tank from piping).
at OIL TANK LEAK / FAILURE CAUSES we provide details about sources of corrosion in underground oil storage tanks and in their piping & connections.
Oil Storage Tank Corrosion Protection Standards
- U.S. Environmental Protection Agency EPA Underground Oil Storage Tank Regulations include - Complete 40 CFR part 280 Technical Standards and Corrective Action Requirements for Owners and Operators of Underground Storage Tanks (UST) (454K byte PDF). This document defines dielectric material (coatings) used to protect underground oil storage tanks from corrosion: More about the galvanic scale and corrosion between dissimilar metals is at GALVANIC SCALE & METAL CORROSION.
- API Recommended Practice 1632, "Cathodic Protection of Underground Petroleum Storage Tanks and Piping Systems"
- NACE RP 0169, "Standard Recommended Practice: Control of External Corrosion on Underground or Submerged Metallic Piping Systems"
- NACE RP 0285 "Standard Recommended Practice: Corrosion Control of Underground Storage Tank Systems by Cathodic Protection"
- NACE Test Method TM 0101, "Measurement Techniques Related to Criteria for Cathodic Protection on Underground or Submerged Metallic Tank Systems"
- NACE Test Method TM 0497, "Measurement Techniques Related to Criteria for Cathodic Protection on Underground or Submerged Metallic Piping Systems"
- STI R892, "Recommended Practice for Corrosion Protection of Underground Piping Networks Associated with Liquid Storage and Dispensing Systems"
- STI-R-972, "Recommended Practice for the Installation of Supplemental Anodes for STI-P3 USTs"
- UL 1746, Standard for Safety: "External Corrosion Protection Systems for Steel Underground Storage Tanks"
...
...
Continue reading at ANODES & DIP TUBES on WATER HEATERS or select a topic from the closely-related articles below, or see the complete ARTICLE INDEX.
Or see these
Recommended Articles
- ALUMINUM WIRING HAZARDS
- ANODES & DIP TUBES on WATER HEATERS
- BLOWER LEAKS, RUST & MOLD
- BOILER LEAKS CORROSION STAINS
- CHIMNEY WET TIME
- COPPER PIPING in BUILDINGS
- CORROSION in ELECTRICAL PANELS
- CORROSIVITY or ACIDITY of WATER
- DIAELECTRIC PIPE FITTINGS
- ELECTRICAL GROUND PIPE CORROSION
- GALVANIC SCALE & METAL CORROSION
- GALVANIZED STEEL WATER PIPING
- GALVANIZED IRON PIPE INSTALLATION
- METAL ROOFING
- OIL TANK LEAK / FAILURE CAUSES
Suggested citation for this web page
GALVANIC SCALE & METAL CORROSION at InspectApedia.com - online encyclopedia of building & environmental inspection, testing, diagnosis, repair, & problem prevention advice.
Or see this
INDEX to RELATED ARTICLES: ARTICLE INDEX to BUILDING FORENSICS
Or use the SEARCH BOX found below to Ask a Question or Search InspectApedia
Ask a Question or Search InspectApedia
Try the search box just below, or if you prefer, post a question or comment in the Comments box below and we will respond promptly.
Search the InspectApedia website
Note: appearance of your Comment below may be delayed: if your comment contains an image, photograph, web link, or text that looks to the software as if it might be a web link, your posting will appear after it has been approved by a moderator. Apologies for the delay.
Only one image can be added per comment but you can post as many comments, and therefore images, as you like.
You will not receive a notification when a response to your question has been posted.
Please bookmark this page to make it easy for you to check back for our response.
IF above you see "Comment Form is loading comments..." then COMMENT BOX - countable.ca / bawkbox.com IS NOT WORKING.
In any case you are welcome to send an email directly to us at InspectApedia.com at editor@inspectApedia.com
We'll reply to you directly. Please help us help you by noting, in your email, the URL of the InspectApedia page where you wanted to comment.
Citations & References
In addition to any citations in the article above, a full list is available on request.
- Borenstein, S. Microbiologically influenced corrosion handbook. Elsevier, 1994.
- Mansfeld, F., D. H. Hengstenberg, and J. V. Kenkel. "Galvanic Corrosion of Al Alloys I. Effect of Dissimilar Metal." Corrosion 30, no. 10 (1974): 343-353.
Abstract: Galvanic interaction between the Al alloys 1100, 2024, 2219, 6061, 7075, and Ag, Cu, Ni, Sn, Cd, Zn, the stainless steels 301, 304L, 347, A286, PH13-8Mo, Steel 4130, Inconel 718, Haynes 188 and Ti-6AI-4V has been studied in air saturated 3.5% NaCl by weight loss measurements and continuous monitoring of the galvanic current in 24 hour tests.
Results show that the potential difference of uncoupled dissimilar metals, while in most cases accurately predicting the direction of current flow, is a poor indicator of the extent (rate) of galvanic corrosion of coupled dissimilar materials.
The values of the average galvanic current density agree well with the increase of dissolution rates due to galvanic coupling. In general, galvanic corrosion of Al alloys coupled to dissimilar metals decreases in the order Ag > Cu > steel 4130 ≫ stainless steels ≈ Ni > Inconel 718 ≫ Ti-6AI-4V ≈ Haynes 188 >Sn > Cd. Coupling to zinc does not result in cathodic protection for all Al alloys studied, but can lead to increased attack as shown by weight loss data.
Galvanic series for each Al alloy based on galvanic current data have been established.
The results of all 95 couples have also been ranked according to the absolute increase of dissolution rates which is proportional to the average galvanic current density (cd). A ranking based on the relative increase of dissolution rates shows pronounced susceptibility to galvanic corrosion for the Al alloys 1100 and 6061, which have low corrosion rates when not in contact with dissimilar metals.
According to the relative increase of dissolution rates which the materials studied cause to Al alloys, they have been placed in three clases (compatible, borderline, and incompatible with Al alloys). - North, R. F., and M. J. Pryor. "The influence of corrosion product structure on the corrosion rate of Cu-Ni alloys." Corrosion Science 10, no. 5 (1970): 297-311.
- Jia, Jimmy X., Guangling Song, and Andrej Atrens. "Influence of geometry on galvanic corrosion of AZ91D coupled to steel." Corrosion Science 48, no. 8 (2006): 2133-2153.
- Sarin, P., V. L. Snoeyink, J. Bebee, W. M. Kriven, and J. A. Clement. "Physico-chemical characteristics of corrosion scales in old iron pipes." Water Research 35, no. 12 (2001): 2961-2969.
- Sarin, P., V. L. Snoeyink, J. Bebee, K. K. Jim, M. A. Beckett, W. M. Kriven, and J. A. Clement. "Iron release from corroded iron pipes in drinking water distribution systems: effect of dissolved oxygen." Water Research 38, no. 5 (2004): 1259-1269.
- Waldeman, Jonathan, "Rust: The Longest War", [at Amazon.com], Simon & Schuster, (2015), ISBN-10: 1451691599,
ISBN-13: 978-1451691597
Excerpt: Rust has knocked down bridges, killing dozens. It’s killed at least a handful of people at nuclear power plants, nearly caused reactor meltdowns, and challenged those storing nuclear waste.
At the height of the Cold War, it turned our most powerful nukes into duds. Dealing with it has shut down the nation’s largest oil pipeline, bringing about negotiations with OPEC.
It’s rendered military jets and ships unfit for service, caused the crash of an F-16 and a Huey, and torn apart the fuselage of a commercial plane midflight. In the 1970s, it was implicated in a number of house fires, when, as copper prices shot up, electricians resorted to aluminum wires.
More recently, in the “typhoid Mary of corrosion,” furnaces in Virginia houses failed as a result of Chinese drywall that contained strontium sulfide. They rusted out in two years. One hundred fifty years after massive ten-inch cast iron guns attacked Fort Sumter, rust is counterattacking.
Union forces have mobilized with marine-grade epoxy and humidity sensors.
Rust slows down container ships before stopping them entirely by aiding in the untimely removal of their propellers. It causes hundreds of explosions in manholes, blows up washing machines, and launches water heaters through the roof, sky high.
It clogs the nozzles of fire sprinkler heads: a double whammy for oxidation. It damages fuel tanks and then engines. It seizes up weapons, manhandles mufflers, destroys highway guardrails, and spreads like a cancer in concrete. It’s opened up crypts. - Zhang, Xiaoge Gregory. Corrosion and electrochemistry of zinc. Springer Science & Business Media, 2013.
- Zhang, X. G. "Galvanic corrosion." Uhlig's Corrosion Handbook 51 (2011): 123.
- Zhang, Zhe, Janet E. Stout, L. Yu Victor, and Radisav Vidic. "Effect of pipe corrosion scales on chlorine dioxide consumption in drinking water distribution systems." Water research 42, no. 1 (2008): 129-136.
- Zhang, X. G. "Galvanic corrosion of zinc and its alloys." Journal of the Electrochemical Society 143, no. 4 (1996): 1472-1484.
- Zhang, X. G., and E. M. Valeriote. "Galvanic protection of steel and galvanic corrosion of zinc under thin layer electrolytes." Corrosion science 34, no. 12 (1993): 1957-1972.
- ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA, 19428-2959 USA The ASTM standards listed below can be purchased in fulltext directly from http://www.astm.org/
- NRCA, Smith, Thomas L., AIA, CRC, STEEL [ROOF] DECK ISSUES for the 1990s [PDF], NRCA, http://www.nrca.net/
- NRCA, Smith, Thomas L., AIA, CRC, STEEL [ROOF] DECK CORROSION BULLETIN [PDF] Professional Roofing, [date pending] p. 58, NRCA, http://www.nrca.net/
- "The Fight Against Corrosion - A Study of the Nature of Corrosion and its Problems in Water Services and Heating Systems", Daniel Davies, Research and Development Services, Stansted Mountfichet, Essex, England, World Plumbing Conference-IV, "Plumbing and the World Environment, Compendium of Workshop Papers, October 3-6, 1996, Hyatt Regency Chicago, Chicago, IL", [personal correspondence, DJF - Author, July 2011]
- NRCA, Smith, T. THE MANY ASPECTS of METAL [ROOF] SHINGLES [PDF] Thomas L. Smith, AIA, CRC., Professional Roofing, [date pending] NRCA, Web: nrca.net
- Building Pathology, Deterioration, Diagnostics, and Intervention, Samuel Y. Harris, P.E., AIA, Esq., ISBN 0-471-33172-4, John Wiley & Sons, 2001 [General building science-DF] ISBN-10: 0471331724 ISBN-13: 978-0471331728
- Building Pathology: Principles and Practice, David Watt, Wiley-Blackwell; 2 edition (March 7, 2008) ISBN-10: 1405161035 ISBN-13: 978-1405161039
- Copper Roofing, by CDA
- Copper Roofing, Master specifications for copper roofing and sheet metal work in building construction: Institutional, commercial, industrial, I.E. Anderson, 1961 (hard to find)
- Corrugated Iron, Building on the Frontier, Simon Holloway
- Historic Preservation Technology: A Primer, Robert A. Young, Wiley (March 21, 2008) ISBN-10: 0471788368 ISBN-13: 978-0471788362
- Metal Roofing, an Illustrated Guide, R.A. Knowlton , [metal shingle roofs],
- In addition to citations & references found in this article, see the research citations given at the end of the related articles found at our suggested
CONTINUE READING or RECOMMENDED ARTICLES.
- Carson, Dunlop & Associates Ltd., 120 Carlton Street Suite 407, Toronto ON M5A 4K2. Tel: (416) 964-9415 1-800-268-7070 Email: info@carsondunlop.com. Alan Carson is a past president of ASHI, the American Society of Home Inspectors.
Thanks to Alan Carson and Bob Dunlop, for permission for InspectAPedia to use text excerpts from The HOME REFERENCE BOOK - the Encyclopedia of Homes and to use illustrations from The ILLUSTRATED HOME .
Carson Dunlop Associates provides extensive home inspection education and report writing material. In gratitude we provide links to tsome Carson Dunlop Associates products and services.