 Gas Furnaces and Indoor Air Quality
Gas Furnaces and Indoor Air Quality
- POST a QUESTION or COMMENT about the impact of gas fired heating furnaces on indoor air pollutants, contaminants, air qualty, or carbon monoxide hazrds
Residential gas heating furnaces impact on indoor air quality:
This article describes possible contributions of a gas fired forced air heating furnace to indoor air levels of carbon monoxide or other contaminants and pollutants. As houses are made increasingly energy efficient, heating system combustion byproducts become important health considerations. High on the list of pollutants is carbon monoxide (CO) an odorless, tasteless toxic gas.
In 1982 the U.S. Consumer Products Safety Commission reported 340 carbon monoxide deaths; 290 (85%) resulted from gas appliances in the home. At the end of this article we explain that residential heating furnaces (and air conditioners) properly installed, maintained, and used, can significantly improve indoor air quality in homes.
InspectAPedia tolerates no conflicts of interest. We have no relationship with advertisers, products, or services discussed at this website.
- Daniel Friedman, Publisher/Editor/Author - See WHO ARE WE?
Gas Furnaces and Indoor Air Quality: Summary of CO Hazards
From 1979 through 1986 the average number of carbon monoxide deaths has dropped to 62 per year. Further, these figures do not reflect the number of families who suffer poor health or who have other complaints ascribed to exposure to carbon monoxide.
[Click to enlarge any image]
A significant number of home inspectors elect to exceed the ASHI Standards of Practice by concerning themselves with this topic.
This article reviews the mechanism of carbon monoxide poisoning, defines acceptable CO levels, and suggests techniques for testing common natural gas furnaces for heat exchanger failures. Carbon monoxide monitors and monitor use are also discussed.
The equipment and methods discussed in this article are based on information from various manufacturers and on the author's experience. This discussion may not be technically complete. Material for this article has been submitted to the American Gas Association and other experts for comment. Feedback will be published in subsequent issues of the ASHI Technical Journal. A Technical Committee review of this paper starts on page 29.>
What Is Carbon Monoxide Poisoning?
 Hemoglobin is an oxygen-bearing protein found in the blood of animals
and man. When hemoglobin combines with carbon monoxide, carboxyhemoglobin
is formed. Carboxyhemoglobin cannot carry oxygen. Therefore, if an
animal or person is exposed to carbon monoxide long enough, the person
will have insufficient levels of hemoglobin-carrying oxygen and serious
illness or death can result.
Hemoglobin is an oxygen-bearing protein found in the blood of animals
and man. When hemoglobin combines with carbon monoxide, carboxyhemoglobin
is formed. Carboxyhemoglobin cannot carry oxygen. Therefore, if an
animal or person is exposed to carbon monoxide long enough, the person
will have insufficient levels of hemoglobin-carrying oxygen and serious
illness or death can result.
Carbon monoxide and hemoglobin have a tremendous attraction to one another and in fact carbon monoxide is absorbed by hemoglobin at 210 times the rate of oxygen absorption.
Blood volume is relative to body mass, small people (especially children) and pets will absorb carbon monoxide and lose their critical oxygen carrying capacity more quickly than large people. People with heart disease who cannot increase the rate of oxygen delivery are candidates for early symptoms of carbon monoxide poisoning.
Carboxyhemoglobin converts back to hemoglobin shortly after exposure, but if exposure is continuous carboxyhemoglobin begins to accumulate. For example, when an individual smokes, exposure lasts several minutes and carboxyhemoglobin converts to hemoglobin prior to the next cigarette. When the smoker has a defective muffler in his car, he gets carbon monoxide from two sources. When the smoker has a bad muffler and sits in a traffic jam morning and evening and goes home to a defective furnace, carboxyhemoglobin levels accumulate.
It is common for families with a defective furnace to suffer headaches, nausea, fatigue, poor concentration, sleep disturbance and palpitations. Such a family will cease to thrive but the failure to thrive will occur well before the family visits a doctor.
 Other air quality
problems, such as inadequate makeup air, can cause complaints but are
not the subject of this paper.
Other air quality
problems, such as inadequate makeup air, can cause complaints but are
not the subject of this paper.
In addition to carbon monoxide, natural gas combustion produces formaldehyde (HCHO). Formaldehyde is similar to carbon monoxide's molecular structure except it contains additional hydrogen atoms. Formaldehyde is a colorless gas that can cause nausea, watery eyes and burning sensations in the eyes and throat.
Irritation from formaldehyde starts at a far lower level than carbon
monoxide. By comparison, the industrial level for exposure to carbon
monoxide is 50 parts per million (ppm) for a healthy worker for 8
hours and exposure to formaldehyde is 3 ppm. To convert between % and ppm
see CONVERT PPM to % CONCENTRATION
Formaldehyde irritates most people at about 1 ppm and many at .5 ppm. Industrial standards are far above the level at which health is affected by both gases.
Furnaces which contribute very low levels of carbon monoxide into a home most likely contribute formaldehyde gas. The potential for formaldehyde gas makes the lowest levels of carbon monoxide a serious warning.
Acceptable Carbon Monoxide Levels in buildings
Carbon Monoxide is odorless, tasteless and leaves the blood within
hours after exposure ends; factors which make carbon monoxide extremely
difficult to study. In order to address the question of how much is too much, the limits
published by government and health organizations are shown in Table
1.
See CO EXPOSURE LIMITS for details.
Table I. Current Carbon Monoxide Exposure Limits in Buildings |
||
| PPM CO Exposure | Effects of Exposure to Carbon Monoxide at this level |
Source/comment |
| 9 ppm | Maximum allowable short term exposure | ASHRAE |
| 10 - 24 ppm | Investigation needed to find source; | Health effects on humans uncertain. |
| 25 ppm | Maximum allowable TWA exposure limit | OSHA. Used in personal CO alarms. |
| 35 ppm | Maximum allowable workplace exposure limit for an 8-hour work shift | NIOSH (40 hour work week) |
| 50 ppm | Maximum allowable workplace exposure limit for an 8-hour work shift | OSHA (40 hour work week) |
| 125 ppm | Workplace alarm must sound | OSHA |
Notes: This table is excerpted from a complete data set found at EXPOSURE LIMITS for CO - Carbon monoxide gas exposure limits - Carbon monoxide exposure limits PEL and TLV set by OSHA and NIOSH. Take note that these standards are industrial, set for the workplace, and were not formulated specifically for residences or home heating systems. For more details also see:
|
||
The standards in the table were published only after scientific data was produced. It is notable that all standards have footnotes describing the limits of knowledge or the limits of research. The footnotes point to the newness of carbon monoxide issues in our homes.
These limits of knowledge and research are reflected in the position of the American Society of Heating, Refrigeration and Air Conditioning Engineers (ASHRAE). In 1984, ASHRAE published a standard of 5 ppm as the acceptable level for carbon monoxide in an indoor environment. In 1989 ASHRAE deleted the standard. The reason for the deletion was that ASHRAE's standard was arbitrary, not based on scientific study and that other published standards varied notably. Hence the ASHRAE standard contributed little to the public well-being.
The national ambient air quality standard, set by the Environmental Protection Agency (EPA), of 9 ppm is based on studies of nervous system and heart changes after exposure to carbon monoxide. Some controversy has resulted from these standards and more study seemed necessary.
Subsequently, the EPA funded a 1989 medical study of treadmill performance and electrocardiogram changes during carbon monoxide exposure performed by the St. Louis University Medical School. In that study heart patients reported pain early in treadmill tests after exposure to carbon monoxide.
The higher the carbon monoxide, the sooner pain and electrocardiogram
changes began. The study concluded that low levels of carbon monoxide
(below 9 ppm) affected treadmill and electrocardiogram performance.
In an accompanying editorial, researchers stated that the safest level
would be the lowest level achievable. (To convert between % and ppm
see CONVERT PPM to % CONCENTRATION)
 Researchers stated that exposure to carbon monoxide for 8 hours at
a rate of 9 ppm allows enough carbon monoxide to take 2 percent of
the body's hemoglobin and these levels result in measurable changes
in the body.
Researchers stated that exposure to carbon monoxide for 8 hours at
a rate of 9 ppm allows enough carbon monoxide to take 2 percent of
the body's hemoglobin and these levels result in measurable changes
in the body.
As a practical matter it is never desirable for a furnace to contribute carbon monoxide to a home. The American National Standards Institute (ANSI) holds as its standards that furnaces shall not contribute exhaust (carbon monoxide) to the room environment.
Figure 1 at left.
The Properties of Natural Gas Natural gas occurs below the earths surface as the result of decomposition of organic matter which may or may not be associated with petroleum. Natural gas is composed of methane (CH4) about 80% to 95%, ethane (C2H6) about 5% to 15% and methyl mercaptan, an odorant, about .1-.3%.
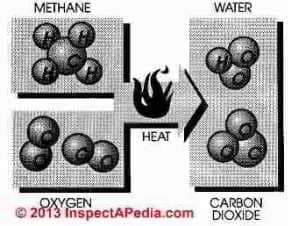
Natural gas is burned by combining gas with air (oxygen) in an exact ratio of 10 parts air to 1 part gas. In theory, when burned, pure methane (CH4) combined with oxygen (O) will produce three products; heat, carbon dioxide (CO2) and water (H20)
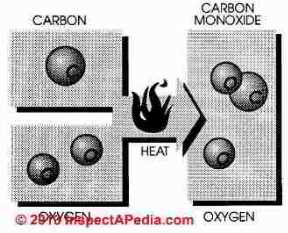 Hence it is easy to imagine
that natural gas is perfectly clean, safe and non-toxic.
Hence it is easy to imagine
that natural gas is perfectly clean, safe and non-toxic.
In practice several factors are not so simple as perfect combustion. We do not burn pure oxygen, we burn air containing oxygen at 20% and nitrogen at 80%. We don't burn methane, we burn methane, ethane, and mercaptan with oxygen and nitrogen.
Figure 2 at left.
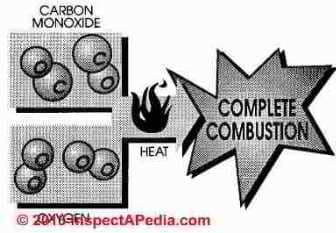
Carbon monoxide is a combustible gas formed by incomplete combustion of hydrocarbon fuel. In perfect combustion one atom of carbon combines with two atoms of oxygen and the end product after combustion is carbon dioxide (CO2). If one atom is missing from the formula, the end product is carbon monoxide
Carbon monoxide is combustible, however, more heat and oxygen must be added to burn carbon monoxide and a cool burning gas furnace leaves notable quantities of carbon monoxide unburned.
In natural gas combustion the final exhaust products will be carbon dioxide and water.
But if oxygen is in short supply carbon monoxide, hydrogen, hydrocarbons, and free carbon will be the final products of combustion. If combustion is incomplete or if mixing of fuel and air are incomplete or the temperature too high or low, a succession of chemical compounds will result. These compounds are called aldehydes and include formaldehyde.
Gas furnaces have a complex exhaust, however, furnace mechanics deal only with carbon monoxide emissions when tuning a burner.
Mechanics
strive to tune furnaces such that the exhaust which flows to the rooftop
contains less than 100 ppm carbon monoxide, but exhaust may be laden
with nitrogen dioxide (NO2), sulfur dioxide (SO2)
and formaldehyde, all of which have undesirable health effects.
Gas Furnace Risk of Releasing Combustion Gases into Indoor Air
The following is a description of the traditional natural gas furnace found in the majority of homes
Gas furnaces manufactured after 1972 may not be as simple as the earlier furnaces because new furnaces have become more complex in order to achieve higher efficiency.
The simplest old furnaces and the new furnaces share the same potential to mix exhaust gases with room air and are monitored for heat exchanger failure using the same inspection method.
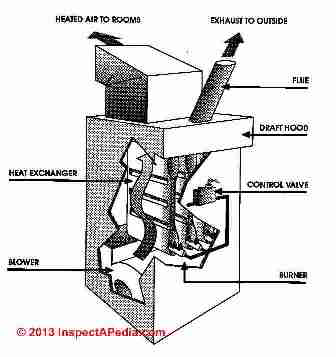
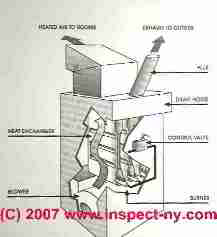
First, the control valve simply allows the gas to flow from the gas piping system through the control valve and into the burner area. The gas passes into the burner through several ports or openings where the gas is mixed with the room air.
These tubes are arranged inside the furnace heat exchanger. At the exterior of the furnace is either an electrical ignition device or a conventional (continuous) pilot light which ignites the gas-air mixture.
The flame rises vertically into the heat exchanger from each burner opening. The heat exchanger is open at the bottom to allow fuel into the combustion area and it is open at the top to allow the exhaust from the combustion process to be collected in a draft hood, routed to the flue and up to the rooftop of the house into open air.
Heat exchangers operate at about 1600 degF. Comparatively speaking 1600 degF is cool - hence the heat exchanger is thin enough to allow temperatures near its exterior to rise to about 400 degF. There room air is circulated by the blower through the openings between the bellows of the heat exchanger and onwards into the rooms of the house as warm air of 100 degF to 120 degF.
This is the critical area of a natural gas furnace. The heat exchanger must be thin enough to allow for the transfer of heat from the open flame, a thickness of about 3/32 of an inch. But also the heat exchanger must be durable enough to withstand heating and cooling multiple times each day for the life of the furnace.
Heat Exchanger Failures
 A heat exchanger in the traditional furnace is made from rolled steel
of two mirror image parts seamed together like a clam shell. Many
furnaces fail by developing cracks in the sheet metal, cracks along
welded seams, or holes due to rust or corrosion.
A heat exchanger in the traditional furnace is made from rolled steel
of two mirror image parts seamed together like a clam shell. Many
furnaces fail by developing cracks in the sheet metal, cracks along
welded seams, or holes due to rust or corrosion.
Occasionally holes formed by rust can be seen with the eye or with the aid of a mirror, but only 20% of the total surface of the heat exchanger is visible to view even with a mirror. Holes or cracks often are visible only when thermal expansion causes the cracks to open. Visual inspection is not normally possible when the furnace is in operation.
Many heat exchangers fail by becoming overheated.
See FURNACE HEAT EXCHANGER LIFE
A heat exchanger is protected from overheating by a carefully adjusted upper limit device. The upper limit device causes the furnace to cycle to its off position when the temperature of the air in the plenum above the furnace exceed the limit set by the mechanic.
A notable number of heat exchangers fail from abnormal rust accelerated by the presence of chlorinated compounds. A chlorinated compound is any compound to which a chlorine molecule is attached. Many household products are chlorinated, among them; solvent, paint thinners, detergents and bleach.
When these compounds mix with humidity, hydrochloric acid is formed and is drawn into the furnace where the acid produces rust and salt deposits. The salt deposits re-combine with moisture from the air in garages, basements and crawl spaces to continue the corrosive process and rapidly ruin a heat exchanger.
Plugged air filters accelerate heat exchanger failure. A furnace filter neglected for several heating seasons will block the flow of air through the heat exchanger. The internal temperature of the furnace may exceed the continuous operating design temperature without reaching the high limit. Broken welds and cracks may result.
Carbon Monoxide Monitors

A carbon monoxide monitor is a sophisticated electronic device which functions by exposing carbon monoxide to two electrodes. If the two electrodes contact carbon monoxide, electrical current will flow between them in direct proportion to the carbon monoxide concentration.
The signal from the electronic cell is amplified and displayed on a liquid crystal display (LCD).
Carbon monoxide monitors are regulated by the Occupational Safety and Health Administration (OSHA) and their range (threshold of sensitivity) and accuracy (stated as a percentage of error) are readily advertised by their manufacturers.
The best equipment is sensitive to concentrations from 0-2000 ppm and are accurate to within 1-3 ppm.
Details are
at CO DETECTION OPTIONS - separate article
Carbon Monoxide Monitoring at Gas Furnaces
 Using a carbon monoxide monitor requires that
a base number be established prior to sampling furnace air.
Using a carbon monoxide monitor requires that
a base number be established prior to sampling furnace air.
The base reading will be the carbon monoxide in the outdoor air prior to entering the house.
A second reading will be taken in the house to demonstrate
that cooking, fireplaces and cigarettes are not altering the base
number.
See GAS DETECTION INSTRUMENTS
At the firing of the furnace, it is important to sample the air at the front of the furnace not at the flue. Flue gas spillage at chimney/draft hood at initial startup (30 seconds) is common. Flue gas spillage from blocked chimneys is a life and death issue, but is not a heat exchanger failure. Don't confuse these gas sources.
Room monitoring should continue through a heating cycle and after the burner has shut down. Many furnaces produce readings only after they have become hot enough to open cracks by thermal expansion.
Early heat exchanger failure produces minor carbon monoxide contamination which can be detected by monitoring high in the room to read the content of the warm exhaust against the ceiling and comparing the reading to the cool air near the floor containing less exhaust and hence, less carbon monoxide.
After the burner is shut down the combustion draft will no longer pull exhaust into the flue and chimney effectively.
 The fan, by blowing
house air over holes in the exterior of the heat exchanger, may then
create a draft drawing residual carbon monoxide into the room.
The fan, by blowing
house air over holes in the exterior of the heat exchanger, may then
create a draft drawing residual carbon monoxide into the room.
Figure 5. at left.
These monitoring techniques will identify early failure where small quantities of 2-3 ppm are in evidence. Grossly damaged heat exchangers may admit 10-30 ppm in the room air in one cycle of the furnace.
As a result of the energy crisis of the 1970s, houses have become increasingly resistant to air changes. Indoor air quality has become an important consideration.
Fortunately, during the same period, technological improvements have made testing instruments available and affordable. Consequently, carbon monoxide detection is becoming a common procedure in the home inspection profession.
How a Furnace Has Positive Effects on Building Indoor Air Quality
Addendum, DJF
Mr. Matzen's article above quite aptly focuses on the dangers of release of combustion gases indoors, particularly carbon monoxide which can be fatal. But reading this article alone can give a mistaken impression about the role that central heating or air conditioning systems can and should play in indoor air quality
. INDOOR AIR QUALITY IMPROVEMENT GUIDE- discusses the full range of steps one can take to improve indoor air in buildings. Here are some details focusing on the positive role of air conditioners, heat pumps, and forced air heating furnaces on indoor air quality.
Heating and cooling system air filters improve indoor particulate levels
In a central air handling system for air conditioning or heating, proper selection and maintenance of air filters, for example, will far outperform individual free-standing "room air cleaners' or "air purifiers" and can actually make a significant reduction in the level of indoor dust and particulates, including allergens and even airborne indoor mold. (Naturally the complete and proper approach is also to find and remove the sources of indoor mold or allergens).
- AIR FILTER EFFICIENCY- discusses the ability of HVAC air filters to remove ultra fine particulates down to the 1-micron level (smallest mold spores, for example)
- AIR FILTERS, OPTIMUM INDOOR- discusses the recommendations for using air filters to improve indoor air quality
To take maximum advantage of centralized supply and return ductwork, air handler and air filtration, in some buildings we operate the blower fan continuously, varying the speed depending on heating or cooling requirements, but continuing fan operation even when heating or cooling are not operating. The result, tested in our forensic laboratory, can drop indoor dust levels substantially. The same system can also provide fresh air or "makeup air" for residential buildings, just as it is required and standard practice for commercial buildings.
- BLOWER FAN CONTINUOUS OPERATION- discusses using the HVAC system to continuously clean indoor air of particulates and in some cases even gases.
- VENTILATION DESIGN PROBLEMS & SOLUTIONS- discusses approaches to building ventilation including fresh air supply in balanced ventilation systems to minimize energy costs
Heating and cooling system air filters improve indoor humidity levels
Air conditioning systems can maintain indoor humidity at the proper level, reducing excessive humidity that contributes to bacterial, dust mite, mold and other indoor airborne irritants during the cooling season. (Some heating systems also provide centralized humidification for the more dry heating season, though we warn that an improperly-located or maintained humidifier risks leaks and damage to the heating equipment.)
See these articles for more in-depth information of how heating and cooling equipment can be used to significantly improve indoor air quality in buildings.
- HUMIDITY LEVEL TARGET - discusses the desired indoor humidity levels in buildings
References
- American Gas Association and the National Fire Protection Association, National Fuel Gas Code, Quincy MA (1988).
- American Society of Heating, Refrigerating and Air Conditioning Engineers, Inc., ASHRAE, ASHRAE Standard 62-1989, Atlanta GA (1989).
- American Society of Heating, Refrigerating and Air Conditioning Engineers, Inc.,
- ASHRAE Handbook-Equipment, Atlanta GA (1988).
- The North American Manufacturing Company, North
American Combustion Handbook, Cleveland OH (1952).
- EPA Indoor Air Division, The Inside Story - A Guide to Indoor Air Quality, Washington D.C. (1988).
- U.S. Consumer Product Safety Commission, Carbon Monoxide Poisoning Involving Vented Gas Space Heaters, Washington D.C. November 1982.
- John N. Kirkpatrick M.D., Occult Carbon Monoxide Poisoning,
- Western Journal of Medicine (January 1987). [Of particular interest regarding effects of chronic exposure to flue gases.]
- American Gas Assoc., Fundamentals of Gas Combustion, Arlington VA (1985).
- American Gas Assoc. Fundamentals of Gas Appliances, (1976).
- Ernest J. Oppenheimer, PhD., Natural Gas: The New Energy Leader
- Pen and Podium Productions. New York NY (1981).
- National Indoor Environmental Institute, Your House and Your Health, Plymouth Meeting PA (1983).
- Elizabeth W. Allred, Et al., Short Term Effects of Carbon Monoxide Exposure on the Exercise Performance of Subjects With Coronary Artery Disease,
- The New England Journal of Medicine, (November 23, 1989).
Adapted from Mr. Matzen's article "Gas Furnaces and Indoor Air Quality", The ASHI Technical Journal, Vol. 2 No. 1, July1991.[1] The original article was submitted to Doug DeWerth and the AGA and to other ASHI reviewers for information and comment as well. As of publication of the 1991 issue of the Journal neither group had replied to the other's paper.
Richard Matzen is a member of the American Society of Home Inspectors in Seattle, WA. He is a frequent writer and lecturer on the topics with which this article is concerned. Illustrations were prepared by Baadh Design, Seattle, imported and scaled for use in this article by the Journal.
...
Continue reading at COMBUSTION PRODUCTS & IAQ or select a topic from the closely-related articles below, or see the complete ARTICLE INDEX.
Or see these
Recommended Articles
Suggested citation for this web page
FURNACES & IAQ at InspectApedia.com - online encyclopedia of building & environmental inspection, testing, diagnosis, repair, & problem prevention advice.
Or see this
INDEX to RELATED ARTICLES: ARTICLE INDEX to GAS HAZARDS in BUILDINGS
Or use the SEARCH BOX found below to Ask a Question or Search InspectApedia
Ask a Question or Search InspectApedia
Try the search box just below, or if you prefer, post a question or comment in the Comments box below and we will respond promptly.
Search the InspectApedia website
Note: appearance of your Comment below may be delayed: if your comment contains an image, photograph, web link, or text that looks to the software as if it might be a web link, your posting will appear after it has been approved by a moderator. Apologies for the delay.
Only one image can be added per comment but you can post as many comments, and therefore images, as you like.
You will not receive a notification when a response to your question has been posted.
Please bookmark this page to make it easy for you to check back for our response.
IF above you see "Comment Form is loading comments..." then COMMENT BOX - countable.ca / bawkbox.com IS NOT WORKING.
In any case you are welcome to send an email directly to us at InspectApedia.com at editor@inspectApedia.com
We'll reply to you directly. Please help us help you by noting, in your email, the URL of the InspectApedia page where you wanted to comment.
Citations & References
In addition to any citations in the article above, a full list is available on request.
- In addition to citations & references found in this article, see the research citations given at the end of the related articles found at our suggested
CONTINUE READING or RECOMMENDED ARTICLES.
- Carson, Dunlop & Associates Ltd., 120 Carlton Street Suite 407, Toronto ON M5A 4K2. Tel: (416) 964-9415 1-800-268-7070 Email: info@carsondunlop.com. Alan Carson is a past president of ASHI, the American Society of Home Inspectors.
Thanks to Alan Carson and Bob Dunlop, for permission for InspectAPedia to use text excerpts from The HOME REFERENCE BOOK - the Encyclopedia of Homes and to use illustrations from The ILLUSTRATED HOME .
Carson Dunlop Associates provides extensive home inspection education and report writing material. In gratitude we provide links to tsome Carson Dunlop Associates products and services.