 How to Convert Between Percent & Parts Per Million (ppm) Concentrations in Liquids or Gases
How to Convert Between Percent & Parts Per Million (ppm) Concentrations in Liquids or Gases
Formula & table to convert ppm to percent concentration
- POST a QUESTION or COMMENT about calculations of concentrations of gases or gas contaminants in air, such as CO or CO2 or H2S
Convert ppm to percent or percent to ppm:
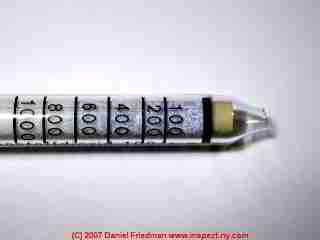
This article describes the simple math used to convert between parts per million (PPM) and percentage, such as how we convert a measurement of 800 ppm of CO2 in air (indicated on our colorimetric gas measurement tube shown at page top) and a percentage concentration of that same gas in air.
InspectAPedia tolerates no conflicts of interest. We have no relationship with advertisers, products, or services discussed at this website.
- Daniel Friedman, Publisher/Editor/Author - See WHO ARE WE?
How to Convert Between Percent of a Gas in Air & Parts Per Million (ppm) of a Gas in Air
Article Contents
- FORMULA for ppm CONVERT to % CONCENTRATION
- FORMULA for % CONCENTRATION to ppm
- DECIMAL POINT LOCATION for ppm to %
- CONVERSION TABLE ppm to / from % CONCENTRATION
- ACCURACY of PPM or % CONCENTRATIONS of GASES in AIR
How to convert ppm concentration to percent concentration
Suppose we are given a parts per million measurement (ppm of X in Y) and we want to convert that to a percent concentration of X in Y.
% Concentration = ppm / 10,000
Percent concentration of substance X within another substance Y = ppm concentration divided by 10,000
or
( Parts per million (ppm) of X ) divided by ( 10,000 ) = Percentage concentration of X in Y
We are calculating the percentage concentration of X in Y even if "Y" is not being stated. People often talk about concentrations without stating the "in what" substance (Y) because they think we know what the heck they're talking about. Or We don't. Or they don't.
If I make a Carbon monoxide (CO) measurement and my instrument tells me I've got 800 ppm of CO, and my hygienist asks me to write the report in percent concentrations what now?
Just watch the decimal point and we can avoid embarrassment.
Example of conversion from parts per million to the percentage concentration of a substance
To convert 800 ppm of gas X to percentage of gas X in air divide the ppm by 10,000
800 / 10,000 = 0.08%
Notice where we place the decimal point if you want to stay out of trouble.
Example of conversion from percentage concentration of a substance to parts per million or ppm
To convert an 8 percent concentration of substance X to parts per million of X (in whatever, air, water, gas)
8.0 % (concentration) x 10,000 = 80,000 ppm
Thanks to Leroy Jenkins for technical editing to clarify this point.
When converting percent concentrations of anything to parts per million remember that a 1 % (concentration) = 10,000 ppm or parts per million.
How to Convert Percent Concentration to PPM or Parts Per Million
This procedure is the opposite of what we just did above. Now we're given a % concentration and we want to express that in parts per million or ppm.
Formula to convert percent concentration into parts per million:
ppm = ( 10,000 ) x ( % concentration )
Where the heck is that 10,000 coming from in these conversion formulas or in the ppm <-> % conversion table we give below?
Remember that 1% means one part in 100 so we can write
1% = 1 / 100
or writing that in decimal form (without the percent) we'd write 1% = 0.01
Remember next that parts one part per million means 1 in a million, so we can write
1 ppm = 1 / 1,000,000
Finally, remember that one percent concentration expressed in parts per million is therefore written as
1% of a million = 0.01 x 1,000,000 = 10,000 ppm
Still nervous about converting between PPM and % concentration? Check your decimal point.
You can always check your math by using 100% - as Shelly Weinberg (statistics & queuing theory instructor at IBM Systems Research Institute) said, "Are you scared of the math, pick a number, any number. Still scared, OK let's try 1. -- or 100%. [3]
Let's try converting 100 percent of something to parts per million.
If we have 100% or "all" of something expressed in parts per million, we should end up with exactly a million parts per million, right?
100% can also be written as 1. But when converting percent concentrations to parts per million we write:
100 x 10,000 = 1,000,000. which is a million (when speaking English).
Where does the decimal point go when converting ppm to percent?
1 part in a million = 1 / 1,000,000 = 0.000001
But to convert that result to a percent number we always multiply by 100. Forgetting this is what makes trouble.
So when we want to convert a parts-per-million number into a percent we divide the number of parts by a million and then multiply that number by 100 to express the same number as a percent "concentration".
If we have just one ppm that can be written as:
1 / 1,000,000 = 0.000001 or one part per million
To convert this raw decimal fraction into a percent we multiply it by 100:
0.000001 x 100 = 0.0001% percent ppm concentration
Or if you like, perform the division and then simply move the decimal point two places over to the right to convert the decimal fraction to a percent concentration.
Is it possible to have more than a 100% concentration of one substance A in another substance B?
No. Since if we get to 100% concentration of substance A, it's taking up 100% of the space and there's no room for the second substance B.
Table of ppm to Percent (%) or Percent Concentration to Parts Per Million
For people who hate multiplying two numbers or who like me are always scared that they put the decimal in the wrong place, here is a table converting between parts per million concentrations and percentage concentrations of anything in anything.
Conversion Table: ppm to Percent (%) or Percent Concentration to Parts Per Million |
|
| Parts per Million - ppm | Percent - % Concentration |
| 0 ppm | 0% |
| 1 ppm | 0.0001% |
| 2 ppm | 0.0002% |
| 3 ppm | 0.0003% |
| 4 ppm | 0.0004% |
| 5 ppm | 0.0005% |
| 6 ppm | 0.0006% |
| 7 ppm | 0.0007% |
| 8 ppm | 0.0008% |
| 9 ppm | 0.0009% |
| 10 ppm | 0.001% |
| 15 ppm | 0.0015% |
| 20 ppm | 0.002% |
| 30 ppm | 0.003% |
| 40 ppm | 0.004% |
| 50 ppm | 0.005% |
| 60 ppm | 0.006% |
| 70 ppm | 0.007% |
| 80 ppm | 0.008% |
| 90 ppm | 0.009% |
| 100 ppm | 0.01% |
| 500 ppm | 0.05% |
| 1,000 ppm | 0.1% |
| 5,000 ppm | 0.5% |
| 10,000 ppm | 1% |
| 20,000 ppm | 2% |
| 30,000 ppm | 3% |
| 40,000 ppm | 4% |
| 50,000 ppm | 5% |
| 60,000 ppm | 6% |
| 70,000 ppm | 7% |
| 80,000 ppm | 8% |
| 90,000 ppm | 9% |
| 100,000 ppm | 10% |
| 200,000 ppm | 20% |
| 300,000 ppm | 30% |
| 400,000 ppm | 40% |
| 500,000 ppm | 50% |
| 600,000 ppm | 60% |
| 700,000 ppm | 70% |
| 800,000 ppm | 80% |
| 900,000 ppm | 90% |
| 1,000,000 ppm | 100% |
Are ppm and % concentration numbers of gas concentrations in air accurate?
Well yeah, in some cases such as measuring gas concentrations in air in an enclosed, stable condition space. Otherwise, well probably not very.
Watch out: in the field when measuring levels of gas contaminants or airborne mold spore contaminants, people are lying to you. Except under controlled environments, the true level of airborne contaminants varies constantly as building conditions, weather, equipment operations, etc. vary.
If you are given an airborne toxic mold spore concentration of 81,926.939 spores per cubic meter of air as a result of a simple "air test for toxic mold", other than a general conclusion that "the number looked pretty big" the rest, the precision is nonsense. It's a very precise number (three decimal places!) but it's almost certainly not truly accurate.
Open a window, turn on a fan, walk through the room, move the sampler, change the time of day, and the number will almost always be enormously different. Not to mention that the most harmful mold in the building might not even have been collected in the air test.
OPINION: I'd prefer to report the number above as "80,000 +/- 50,000" or something like that, based on a combination of experience, building observations, case history, procedures and equipment used.
The "right" answer to such studies is "it looks big" or "it doesn't look big". Why don't your labs and experts report like this? Competition.
More digits = "credibility" in the minds of some customers. "If we don't report that way we'll lose business" is what lab directors have told me at conferences. So it's our fault for not paying attention in 6th grade math class. (Thank you Miss Morton, Tuckahoe Elementary School).
Details of this problem are
at ACCURACY vs PRECISION of MEASUREMENTS.
Example: Concentrations of Carbon Monoxide
Bachrach indicates that for gas-fired equipment CO should not exceed 400 ppm in the flue gases. [1]
Bacharach explains that conversion burners and gas-designed equipment, if over-fired, can have flame impingement on cold surfaces and may produce CO even if they are being run with excess air and even though CO2 and O2 are at acceptable limits.[1][2]
Excess air is introduced in gas combustion to assure that no CO remains in the flue products. S
ee Matzen's FURNACES & IAQ or for an engineer's view
see COMPLETE COMBUSTION, STOICHIOMETRIC for details about "complete combustion".
RW Beckett Corporation also produces combustion and flue-gas measurement equipment. However a review of literature they provided to us did not find equipment specifically for CO measurements. Beckett's tests measure oxygen percentage.[1][2]
Other companies such as MSA, ISC, Monitox, and MDA also produce equipment which may be usable for these tests; these companies did not provide literature for this article.
Details are at CO DETECTION OPTIONS
...
Reader Comments, Questions & Answers About The Article Above
Below you will find questions and answers previously posted on this page at its page bottom reader comment box.
Reader Q&A - also see RECOMMENDED ARTICLES & FAQs
On 2019-07-20 by (mod) - how to express gas concentrations by weight PPMW versus PPMV by volume
Phillip:
We do not normally convert PPM concentrations of a gas by volume into weight for CO2 or other gases; the gas detection tube or gas monitoring device is giving us a direct reading of PPM in the air being tested.
The most common gas concentration measurements are made as PPMV - parts per million volume.
Weight is not calculated.
Weight could be expressed as mg/L or as PPMW - parts per million by weight.
Any by-weight concentration, such as expressed in mg/L or milligrams of a substance (CO2 in your case) per L of volume (presumably of air in your case) requires the gas density as I'll explain.
We would have to make additional measurements of the density of the gases involved to convert to weight as you suggests.
Lentech has a succinct explanation:
"To convert ppmv to a metric expression like µg/m3, the density of the concerning gas is needed. The density of gas can be calculated by the Law of Avogadro's, which says: equal volumes of gases, at the same temperature and pressure, contain the same number of molecules." - retrieved 2019/07/20 original source https://www.lenntech.com/calculators/ppm/converter-parts-per-million.htm
BTW Avogadro's constant will be 6.02 x 1023 - the number of molecules in one gram molecular weight of a substance.
I think one reason we avoid the ppmw or PPMW expression of gas concentrations such as CO2 in air about which you ask is that the density of air and gases is NOT constant but varies continually as temperatures (and perhaps weather and barometric pressure) change.
On 2019-07-20 by Phillip
How do you convert PPMV to actual weight for CO2? What else must be known here?
On 2018-10-21 by (mod) - explain parts per million
ten parts per million of CO in air is exactly that.
To understand "what would be the total composition of gases" in any quantity of air please see details at CONCENTRATIONS of GASES in AIR
On 2018-10-21 by Olumese
Please what would be the total composition of gases in 10 ppm CO in air balance
On 2016-11-08 by (mod) -
Thanks Leroy. Indeed 800/10000 = 0.08 or 8 hundredths which can be re-written as 8%. i.e. 8% = 0.08
At the start of the article above I mis-typed to include a % sign where I shouldn't have. I've edited and corrected the text.
Thank you for the editing help. Working together makes us smarter.
On 2016-11-07 1 by Leroy Jekins
This is wrong. 800/10000 is not .08%. It is 8%. So it would be 80000ppm not 800.
On 2015-06-07 by Carmel Brincau
How should I explain to transport Authorities that reducing Diesel Exhaust Nox from the tail pipe should be enough to upgrade a Diesel engine to Euro 5 EU standard Regulations
...
Continue reading at GAS DETECTION INSTRUMENTS or select a topic from the closely-related articles below, or see the complete ARTICLE INDEX.
Or see these
Recommended Articles
- COLORIMETRIC GAS DETECTION TUBES
- CONVERT PPM to % CONCENTRATION
- Drager GAS DETECTORS
- GAS DETECTION INSTRUMENTS - home
- GAS DETECTOR WARNINGS
- GAS DETECTOR TUBE WARNINGS
- GAUGE, REFRIGERATION PRESSURE TEST
- GAS EXPOSURE LIMITS & STANDARDS - home
- GASES, EXPOSURE, TESTING
- ODORS GASES SMELLS, DIAGNOSIS & CURE
- SMELL PATCH TEST to FIND ODOR SOURCE
- TIF 5000 GAS DETECTOR
- TIF 8800 GAS DETECTOR
Suggested citation for this web page
CONVERT PPM to % CONCENTRATION at InspectApedia.com - online encyclopedia of building & environmental inspection, testing, diagnosis, repair, & problem prevention advice.
Or see this
INDEX to RELATED ARTICLES: ARTICLE INDEX to GAS HAZARDS in BUILDINGS
Or use the SEARCH BOX found below to Ask a Question or Search InspectApedia
Ask a Question or Search InspectApedia
Try the search box just below, or if you prefer, post a question or comment in the Comments box below and we will respond promptly.
Search the InspectApedia website
Note: appearance of your Comment below may be delayed: if your comment contains an image, photograph, web link, or text that looks to the software as if it might be a web link, your posting will appear after it has been approved by a moderator. Apologies for the delay.
Only one image can be added per comment but you can post as many comments, and therefore images, as you like.
You will not receive a notification when a response to your question has been posted.
Please bookmark this page to make it easy for you to check back for our response.
IF above you see "Comment Form is loading comments..." then COMMENT BOX - countable.ca / bawkbox.com IS NOT WORKING.
In any case you are welcome to send an email directly to us at InspectApedia.com at editor@inspectApedia.com
We'll reply to you directly. Please help us help you by noting, in your email, the URL of the InspectApedia page where you wanted to comment.
Citations & References
In addition to any citations in the article above, a full list is available on request.
- In addition to citations & references found in this article, see the research citations given at the end of the related articles found at our suggested
CONTINUE READING or RECOMMENDED ARTICLES.
- Carson, Dunlop & Associates Ltd., 120 Carlton Street Suite 407, Toronto ON M5A 4K2. Tel: (416) 964-9415 1-800-268-7070 Email: info@carsondunlop.com. Alan Carson is a past president of ASHI, the American Society of Home Inspectors.
Thanks to Alan Carson and Bob Dunlop, for permission for InspectAPedia to use text excerpts from The HOME REFERENCE BOOK - the Encyclopedia of Homes and to use illustrations from The ILLUSTRATED HOME .
Carson Dunlop Associates provides extensive home inspection education and report writing material. In gratitude we provide links to tsome Carson Dunlop Associates products and services.

