 Hot Water Expansion & Pressure FAQs
Hot Water Expansion & Pressure FAQs
- POST a QUESTION or COMMENT about the expansion of water or increase of water pressure when water temperature is increased
Questions & answers about thermal expansion in hot water systems:
Frequently-asked questions & answers about the thermal expansion of water as it is heated.
This article series defines thermal expansion in water equipment in response to temperature, and explains the simultaneous increase in system pressure. The artile series also shows how to calculate hot water pressure increase in water heaters, calorifiers, geysers, hot water cylinders and heating boilers as a function of the increase in water temperature.
InspectAPedia tolerates no conflicts of interest. We have no relationship with advertisers, products, or services discussed at this website.
- Daniel Friedman, Publisher/Editor/Author - See WHO ARE WE?
FAQs on the Expansion Rate of Hot Water as it is Heated
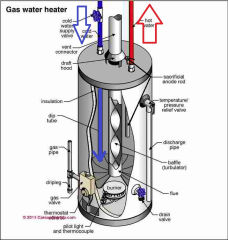 Recently-posted questions & answers about the pressure increase and expansion of hot water - these questions and replies were posted originally
Recently-posted questions & answers about the pressure increase and expansion of hot water - these questions and replies were posted originally
at HOT WATER PRESSURE EXPANSION RATE
Hot water heater or cylinder or calorifier or geyser sketch shown here is provided courtesy of Carson Dunlop Associates, a Toronto home inspection, report writing tool & education company.
[Click to enlarge any image]
On 2017-03-17 by (mod) - How to convert a coefficient of thermal expansion of a material to an expansion rate?
Lerato
The coefficient of expansion gives us the increase in dimension as a change between two temperature points.
An expansion "rate" would, if I am guessing at what you're actually asking, express expansion of a material's dimension over time in response to temperature change.
My suggestion would be to plot the temperature change itself over that time = that is temperature of the material not just ambient temperature as there will be a delay or lag due to thermal mass and the effect of the material being in contact with other materials, sheltered from temperature, wind, sun, etc. (So you see it's complex if you want to do it right)
Once you have the temperature change of your material over time you can simply calculate, based on the coefficient of expansion, just how much the material has changed in dimension over time by correlating it to the temperature change over the same time interval. It's a simple plot.
Be sure to also see THERMAL EXPANSION of MATERIALS where we give the coefficients of thermal expansion for many building materials.
and an interesting application of this approach is
On 2017-03-17 by LERATO
How do I convert a coefficient of thermal expansion of a material to an expansion rate?
On 2016-05-26 by (mod) - what is the change in water pressure in a water tank or pipe when we heat up the water?
P1V1 = P2V2 is the general equation relating the change in pressure as volume changes
From COMBINED GAS LAW in particular where we consider the effect of temperature on water pressure in a closed container, we have
P1V1/T1 = P2V2/T2
You can see that if the volume is not changing (since your water tank or water pipe filled with water (no air is in the space) doesn't change size by any meaningful amount when you heat it), you can simplify this formula FOR JUST THIS CASE to
P1/T1 = P2/T2
From there the math is so easy even I can do it. Let me know if it's giving you trouble.
You will also want to read: why it is the case that when we add temperature, again from the Ideal Gas Equation discussed at,
WATER TANK PRESSURE CALCULATIONS where we review Boyles Law, Charles Law, the combined gas law,
and WATER PRESSURE TANK LAW: why Boyle's, Charle's, and the Combined Gas Law are Wrong about Water Tank Air and Water Pressures.
On 2016-05-26 by Omar al terkaoui
water in closed pipe pressure inside pipe 12 bars [of water pressure]at temperature 25 C volume of water 132 m3 and sunshine the temperature increased to 55 C. What is the new pressure inside the pipe?
On 2016-03-14 by (mod)
I can't guess, Brian. You need to specify a starting temperature; otherwise the "heated to 90 C" isn't telling us enough; that is, starting from 89.9 deg C the water will expand so little you'd not be able to measure it while starting from just above freezing (0 C) is another matter.
Here's the basic calculation you'll need:
The thermal coefficient of expansion of water is 0.00021 per 1 °C at 20 °C
You can see it's not much volumetric expansion (and it's not dead linear either).
On 2016-03-13 by Brian Whitworth
Could you please tell me what volume expansion, would I get on an open system containing 17 litres of water heated to 90 degrees C?
On 2015-12-24 by (mod)
That may be a hissing sound coming from the elements heating water inside, perhaps coated with minerals;
Are you sure there is air coming from the TP valve - that'd be very very odd; the water tank discharges air ONLY when it is being filled from empty. The tank is normally full of water.
On 2015-12-24 by Benjamin
My water heater is about 9 months old. the problem I have is it has a sound as if its releasing air somewhere. It is an electric water heater. I have changed the pressure valve 2 times and still have that air sound coming from it. Why is this?
On 2015-12-03 by (mod)
Ann
You're asking perfectly reasonable questions but this is not a problem that one can safely answer in detail from a simple e-text and knowing almost nothing about the water supply system, piping, controls, etc. I suggest that you ask your water utility for help, including an on-site visit; let me know what you're told and we'll proceed from there.
On 2015-12-03 by Ann Turner
Dear Sir, I have a house building site and road widening near me. I am experiencing low resonance sound waves in the house virtually 24 hours a day. I believe it is from a pressurised drainage pump / pipe and for some reason I am getting the vibrations from this and sound waves when it fills up or drains out.
Someone believes they may have emptied a water pipe and re-routed a pipe to stop the site from flooding. Hence giving me a problem. The water company said the water is 5 bars, which other companies think is too high.
We did have a burst main on the road last week. I am really concerned, could you advise me if it would be possible to empty the pipe under my house or maybe re-route a pipe so that I get this low resonance vibration/sound waves. Please can you help me understand this noise, I am getting rather frightened and it can hurt my ears and gives me headaches. Thank you.
Question: can thermal expansion cause my hot water tank relief valve to drip
I was told that thermal expansion is the reason the temperature & pressure relief valve on my water heater is dripping. Is that OK?
Reply:
No. The cause of the dripping, which could be due to a hot water thermal expansion sysem in a closed system, could also be due to any of a number of other problems.
In all cases a drippy TP valve is potentially dangerous both because we don't yet know the reason for the drip (the system may already be unsafe) and because the safety valve may clog and then fail to protect the system.
See RELIEF VALVE, WATER HEATER.
...
Continue reading at HOT WATER PRESSURE EXPANSION RATE or select a topic from the closely-related articles below, or see the complete ARTICLE INDEX.
Or see these
Recommended Articles
- BLEVE EXPLOSIONS - why water heater tanks or calorifiers might explode if the relief valve fails,
- HOT WATER EXPANSION TANKS
- THERMAL EXPANSION TPR VALVE LEAKS - why the relief valve leaks when heating water
- RELIEF VALVE LEAKS for a diagnosis of all of the causes of leaks at TPR valves.
- THERMAL EXPANSION TPR VALVE LEAKS we explain how normal thermal expansion in a hot water system can cause pressure & temperature relief valve leaks
- WATER HEATER SAFETY
- WATER PRESSURE MEASUREMENT
Suggested citation for this web page
HOT WATER PRESSURE EXPANSION FAQs at InspectApedia.com - online encyclopedia of building & environmental inspection, testing, diagnosis, repair, & problem prevention advice.
Or see this
INDEX to RELATED ARTICLES: ARTICLE INDEX to T&P RELIEF VALVES
Or use the SEARCH BOX found below to Ask a Question or Search InspectApedia
Or see
INDEX to RELATED ARTICLES: ARTICLE INDEX to PLUMBING SYSTEMS
Or use the SEARCH BOX found below to Ask a Question or Search InspectApedia
Ask a Question or Search InspectApedia
Try the search box just below, or if you prefer, post a question or comment in the Comments box below and we will respond promptly.
Search the InspectApedia website
Note: appearance of your Comment below may be delayed: if your comment contains an image, photograph, web link, or text that looks to the software as if it might be a web link, your posting will appear after it has been approved by a moderator. Apologies for the delay.
Only one image can be added per comment but you can post as many comments, and therefore images, as you like.
You will not receive a notification when a response to your question has been posted.
Please bookmark this page to make it easy for you to check back for our response.
IF above you see "Comment Form is loading comments..." then COMMENT BOX - countable.ca / bawkbox.com IS NOT WORKING.
In any case you are welcome to send an email directly to us at InspectApedia.com at editor@inspectApedia.com
We'll reply to you directly. Please help us help you by noting, in your email, the URL of the InspectApedia page where you wanted to comment.
Citations & References
In addition to any citations in the article above, a full list is available on request.
- In addition to citations & references found in this article, see the research citations given at the end of the related articles found at our suggested
CONTINUE READING or RECOMMENDED ARTICLES.
- Carson, Dunlop & Associates Ltd., 120 Carlton Street Suite 407, Toronto ON M5A 4K2. Tel: (416) 964-9415 1-800-268-7070 Email: info@carsondunlop.com. Alan Carson is a past president of ASHI, the American Society of Home Inspectors.
Thanks to Alan Carson and Bob Dunlop, for permission for InspectAPedia to use text excerpts from The HOME REFERENCE BOOK - the Encyclopedia of Homes and to use illustrations from The ILLUSTRATED HOME .
Carson Dunlop Associates provides extensive home inspection education and report writing material. In gratitude we provide links to tsome Carson Dunlop Associates products and services.

