 Validity of Mold Culture Tests
Validity of Mold Culture Tests
- POST a QUESTION or COMMENT about buying and using do-it-yourself mold tests
Mold culture test kit accuracy:
Are culture plates a reliable method to screen buildings for indoor mold contamination? This article discusses building mold tests that rely only on settlement plates or swabs to find toxic mold in buildings. Before you buy any "mold home test kit" for mold you should read this article.
This article explains the limited accuracy of mold cultures when used as "mold test kits" to examine indoor air quality as an investigation methodology in searching for possible causes of respiratory illness, asthma, immune system disorders, rashes, skin disease, psychological and neurological disorders, eye infections, or other symptoms that may have a physiological and environmental component.
InspectAPedia tolerates no conflicts of interest. We have no relationship with advertisers, products, or services discussed at this website.
- Daniel Friedman, Publisher/Editor/Author - See WHO ARE WE?
Problems With Relying on cultured mold samples to evaluate a building
 If you smell mold or see mold in a building, most-often you do not need to "test" for mold contamination. Find and remove the mold, and fix the leaks that caused its growth.
If you smell mold or see mold in a building, most-often you do not need to "test" for mold contamination. Find and remove the mold, and fix the leaks that caused its growth.
A thorough visual inspection by an expert along with a collection of the leak history of a building, occupant indoor air quality complaints, and particular health vulnerabilities of occupants should produce a finding that either asserts that further mold work is not needed OR a report that outlines what mold cleanup and building repairs are needed.
Despite the inaccuracy of such tests, many consumers seek a "mold test" to screen for indoor mold contamination.
Why don't we use readily-available mass-marketed cultures, settlement plates, and swab kits such as those available at the local hardware store?
Watch out: While all "mold tests" and "mold test kits" or mold sampling methods have their limitations, the usefulness of mold culture plates as a general screen for harmful indoor mold contamination is particularly limited, inaccurate, and most-likely to give at best an incomplete picture of the indoor air environment.
OPINION: because of the 100,000 mold species known and the estimated 5.1 million mold genera/species on earth, most mold genera / species - about 90% of them - simply will not grow in any culture. (Haines 2016).
So even if the culture does successfully grow mold we don't know if this accurately represents what mold contaminants are in the building tested.
The underlying methodology of this test may be seriously flawed if you're relying on the results of culturing to characterize just what problematic fungal spores are present in a building.
Mold cultures, typically taken using settlement plates, Anderson-type samplers, and sterile swabs, can be quite unreliable as indicators of what's really present in an indoor environment.
This is especially true if the test does not detect a mold problem (one may be present but was not detected by this method) and it might be true even if the test does seem to detect a problem as it may detect molds other than the largest or most serious mold contamination reservoir that's present.
As another test limitation example, a dead spore in the air may be toxic or pathogenic (containing mycotoxins for example) but that spore may not grow at all in a culture medium.
In addition, variations in building indoor air movement, activity, humidity, temperature, and other conditions causes an enormous variation in the level in air of all sorts of indoor particles. I have found as much as four orders of magnitude in the level of airborne mold spores as these conditions in buildings were changed.
And individual mold spores, varying by size, mass, toxicity, and preference for mold culture, find very different rates of first settlement out of the air onto any building surface (including a culture plate) and second rates of growing within any given culture medium (including a range of growth from abundant to zero).
... culture-based methods likely will not work except for very hardy microbes [Farnsworth et al. 2006]
...Fungal spores have physical diameters of about 0.5 to 30 µm or larger, while the aerodynamic diameters of airborne fungal spores and spore clusters are reported to be from 0.9 to 5 µm [Eduard 2009; Hussein et al. 2013; Reponen et al. 2011b].
You can see that a culture that grows only some mold species can be particularly misleading, even dangerous to rely upon when investigating building-related illness.
Mycotoxin-producing and pathogenic species have to be detected specifically, however, because of their higher toxicity. - Eduard 2009.
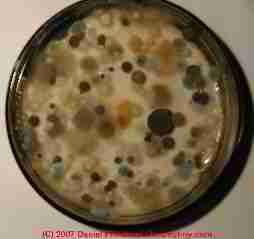
In our mold culture photo above you see a "home test kit" for mold collected in a Washington DC apartment in the Watergate. Apparently there are about seven different mold genera/species appearing on this "overgrown" culture plate.
But the fastest growing molds (those who most like the media) will of course overgrow and hide other mold spores that may have landed on the media. And then, heavier larger spores tend to settle out of air sooner than smaller lighter spores. So the culture plate may over-represent heavy large molds compared with the actual molds present in the building.
Use of cultures as building screens for the presence or absence problematic mold is unreliable - only 10% of all molds of any genera will grow on any culture under any circumstances, so a mold culture screening test for mold is 90% wrong at the outset. More so if one considers that certain molds that can be grown in culture only respond to specific culture media.
Even if a mold is grown on a culture, given these constraints one cannot reliably infer that the mold grown is the problem material in a building. Therefore no screening test by air or culture is an adequate substitute for nor superior to the value of a careful visual inspection by an experienced inspector who knows where mold is likely to grow and what it looks like on or in building surfaces and cavities.
Other serious flaws include inconsistent presence of problematic particles in building air, variations in particle settling rate out of air, variations in growth rates on different media of different mold species (fast growing spore A over-grows and hides the presence of slow growing spore B) and the fact that some problematic spores which could be hazardous to building occupants simply do not grow at all in the culture medium.
There is indeed a valid place for cultures (air or swab) in the arsenal of building investigation tools (cross check on visual inspection and bulk sampling, cross check on clearance inspection and sampling, and elaboration of dormant particles).
Also see SWAB TEST KIT PROCEDURE
Culture methods for fungal spore determination are an important tool, but these methods should not be relied-upon as the principal means for determining what problematic particles are in indoor air.
Relying on over-the-counter home test kits for mold to evaluate a building
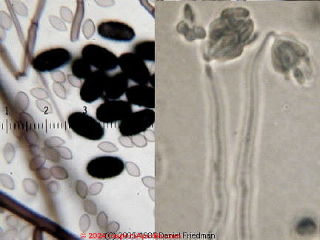
Home test kits for mold are inexpensive, easily available, and easy to use. Therefore we wish we could say they could be an OK place to start, but we don't think this is the most accurate approach to screening a building for mold.
In a recent field experiment we used an over-the-counter "mold test kit" according to its instructions while we also performed a professional inspection of the building.
Among problems which our inspection discovered in the building the settlement-plate culture "toxic mold test kit" successfully found an Aspergillus sp. presence.
It also found some nice Alternaria sp. spores, as well as the usual other collection of common Cladosporium species found in air.
What the mold test kit failed to find was what was probably making the occupants in the building sick. Our visual inspection identified various area of mold on surfaces and in the building cavities.
We collected bulk (tape) samples (as well as vacuum samples (such as vacuum samples of fiberglass building insulation) and we also collected some air samples used as a cross-check screen).
A strategic examination of these samples identified a very extensive Stachybotrys chartarum infection in the building, Penicillium, and an extensive Chaetomium globosum colony as well as the Aspergillus and the less troublesome Alternaria and its buddies.
The first two mold species are toxic, the last, allergenic. They were totally missed by the "test kit."
Why did the home test kit for mold fail to find the actual problem in the building?
In addition to our bulk samples (which found the mold missed by the "home test kit") we also used two different types of air sampling machines as well as pulling some vacuum samples of suspect carpeting in an area which looked pretty clean.
Remarkably, our air samples confirmed the Stachybotrys chartarum presence, a spore not so easily found in air, despite the fact that we did nothing more than walk across a carpeted room during the test.
Mold spores may appear or fail to appear in an air test or "spore trap" for mold because of significant variations in particle disturbance during activity in the building, though there is a huge number of other factors which affect air and particle movement inside.
We provide more details about air movement in buildings
at ACCURACY OF AIR TESTS for MOLD
In this building the owner had begun a do-it-yourself demolition and repair of a water-damaged bathroom. Extensive mold contamination was on the exposed side of bathroom drywall and more extensive mold was growing on the cavity side of these walls.
As the owner used a hammer and hatchet to smash and remove drywall, considerable levels of airborne mold were produced - a condition probably more hazardous to the occupants than when the mold was simply growing on and in surfaces and cavities.
We are often able to spot a building where there has been a previous demolition of moldy materials by examining dust from remote surfaces.
The actual exposure level of the building occupants to this mold is not something one can immediately infer from finding leftover traces in a building, but if professional containment and remediation measures were not followed, there is at least a risk that for a time the occupants may have been breathing some pretty moldy air.
In the case described here, the owner who performed the demolition developed a rather ugly skin rash that appeared to be mold-related, and which abated after a combination of treatment and some proper housecleaning.
Personal Field Experience Finds Wide Variation of Airborne Mold Spores over Short Time Intervals
Really? While Pasanen (1991) found that
The relative humidity of air had no direct influence on the growth of fungi.
By repeated measurements of airborne mold levels of a species of Aspergillus sp. at the same location on successive days during a process of dehumidification in a moldy library basement, I found that the level of airborne Aspergillus sp. spores ranged from barely detected (counts in the tens of spores per liter of air) to very high (tens of thousands of spores per liter of air) as the indoor relative humidity fell.
I posit that the very thick mold visible on books in this historically very damp space consisted in, among others, Aspergillus sp. that began to release its spores at dramatically increased levels as the area began to dry.
Most mold species have not been named nor studied
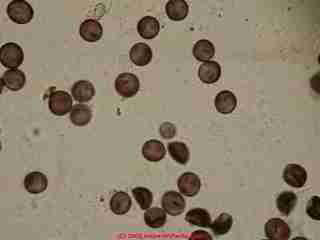 Of over 5 million mold genera/species currently estimated to be growing away on earth, less than 100,000 individuals have been named and studied at all.
Of over 5 million mold genera/species currently estimated to be growing away on earth, less than 100,000 individuals have been named and studied at all.
Less than two percent of all molds have been studied. (Blackwell 2011)
And mold is everywhere, even inside the U.S. Laboratory module of the International Space Station, (Vesper 2008).
Happily for people cleaning up a mold problem or diagnosing a medical or allergenic mold problem, we can do a little better.
There are probably about 200 common mold genera/species that are often found indoors growing in or on building materials.
While there are many others who may make an occasional appearance, even as a large area of mold growth, most often it's one of these 200 or so molds.
Therefore, while in general only about 10% of molds may grow in a culture medium, the number of common indoor molds that can be cultured is probably greater.
Still there are better approaches to screening a building for indoor mold contamination. A visual inspection by an expert is the most-critical service. To support a claim that there was or was not cross-contamination during the subsequent mold remediation job, a few settled dust samples may permit "before and after" mold tests.
Surface Dust & Tape Sampling: An Alternative to Mold Cultures & Speciation for Building Screening Tests
We prefer collecting physical samples of representative settled dust as that will collect both viable and non-viable mold spores. We cannot perform accurate quantitative analysis of a surface or tape sample but we can recognize when there is an unusual level of a particular problem particle, mold or otherwise.
Keep in mind that except for special circumstances (medical need, need to prove that other building dust is due to improper dust containment during a mold remediation) we do not need to know the mold's name to clean it up.
Except for cosmetic (harmless) black mold that we sometimes find on framing lumber, we want the indoor mold to be cleaned-up (removed) and we want the cause for its growth to be corrected.
See also COSMETIC MOLD, RECOGNIZE
Research on Accuracy of Mold Culture Testing for Indoor Mold Contamination
- Ashley, Kevin, Ph.D. and Paula Fey O’Connor, NIOSH, NIOSH MANUAL of ANALYTICAL METHODS (NMAM), 5th Edition, (March 2017) NIOSH, DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Disease Control and Prevention National Institute for Occupational Safety and Health, retrieved 2018/05/17, original source: https://www.cdc.gov/niosh/nmam/pdfs/NMAM_5thEd_EBook.pdf
Excerpts:
Passive bioaerosol sampling can be limited by several variables including the air currents around the device and airborne particle size. As discussed earlier, large particles settle much more quickly than small particles.
Thus, large particles are much more likely to be collected by passive samplers [Haig et al. 2016; Reponen et al. 2011b].
As a result of these limiting variables, results from passive bioaerosol sampling cannot be directly related to the concentration of airborne particles and may not correlate well with results from active sampling [Reponen et al. 2011b].
...
because the results from settle plates cannot be directly compared to the amount of airborne microbes, they should only be used for qualitative, not quantitative, evaluations
...
Many microorganisms lose viability after collection, so although PCR-based methods may be effective, culture-based methods likely will not work except for very hardy microbes [Farnsworth et al. 2006].
...
Viable microorganisms may be culturable or non-culturable. Culturable organisms reproduce under controlled laboratory conditions.
Non-culturable organisms do not reproduce in the laboratory because of intracellular stress or because the conditions (e.g., culture medium or incubation temperature) are not conducive to growth.
...
Only culturable microorganisms are enumerated and identified, thus leading to an underestimation of bioaerosol concentration.
...
Broad viable culture approaches favor species belonging to the phylum Ascomycota, as well as species that outcompete slower-growing species.
Several different types of media and physiological conditions (e.g. temperature) may also need to be employed to assess complete fungal diversity using this approach.
For fungi, selection of the nutrient media may potentially bias the growth of specific viable fungal bioaerosols.
...
Often, it is difficult to identify multiple colonies at one location on a plate because of the lack of differential colony morphology [Burge et al. 1977].
In addition, some organisms produce large, spreading colonies while others produce microcolonies.
Analysis of plates containing multiple types of microorganisms can be difficult because the chemicals secreted by one microorganism might inhibit the growth of other microorganisms at that same location [Burge et al. 1977]. The morphology of the colony of one microorganism also may completely obscure that of another, and a fast-grower might obscure a slowgrower.
...
The collection and classification of nonviable and non-culturable microorganisms cannot be performed by using viable culture methods.
A large proportion of fungal bioaerosols are nonviable and would not grow and proliferate on nutrient media [Eduard et al. 2012].
- Blackwell, Meredith. "The Fungi: 1, 2, 3… 5.1 million species?." American journal of botany 98, no. 3 (2011): 426-438.
Abstract excerpt:
Premise of the study: Fungi are major decomposers in certain ecosystems and essential associates of many organisms. They provide enzymes and drugs and serve as experimental organisms. In 1991, a landmark paper estimated that there are 1.5 million fungi on the Earth.
Because only 70000 fungi had been described at that time, the estimate has been the impetus to search for previously unknown fungi. Fungal habitats include soil, water, and organisms that may harbor large numbers of understudied fungi, estimated to outnumber plants by at least 6 to 1.
More recent estimates based on high-throughput sequencing methods suggest that as many as 5.1 million fungal species exist.
...
Fungi are essential to the survival of many groups of organisms with which they form associations. They also attract attention as predators of invertebrate animals, pathogens of potatoes and rice and humans and bats, killers of frogs and crayfish, producers of secondary metabolites to lower cholesterol, and subjects of prize-winning research.
Molecular tools in use and under development can be used to discover the world’s unknown fungi in less than 1000 years predicted at current new species acquisition rates. - Burge, Harriet A., William R. Solomon, and Michael L. Muilenberg. "Evaluation of indoor plantings as allergen exposure sources." Journal of Allergy and Clinical Immunology 70, no. 2 (1982): 101-108.
- Buttner, Mark P., and LINDA D. Stetzenbach. "Monitoring airborne fungal spores in an experimental indoor environment to evaluate sampling methods and the effects of human activity on air sampling." Applied and environmental microbiology 59, no. 1 (1993): 219-226.
Abstrac:
Aerobiological monitoring was conducted in an experimental room to aid in the development of standardized sampling protocols for airborne microorganisms in the indoor environment.
The objectives of this research were to evaluate the relative efficiencies of selected sampling methods for the retrieval of airborne fungal spores and to determine the effect of human activity on air sampling.
Dry aerosols containing known concentrations of Penicillium chrysogenum spores were generated, and air samples were taken by using Andersen six-stage, Surface Air System, Burkard, and depositional samplers.
The Andersen and Burkard samplers retrieved the highest numbers of spores compared with the measurement standard, an aerodynamic particle sizer located inside the room. Data from paired samplers demonstrated that the Andersen sampler had the highest levels of sensitivity and repeatability.
With a carpet as the source of P. chrysogenum spores, the effects of human activity (walking or vacuuming near the sampling site) on air sampling were also examined.
Air samples were taken under undisturbed conditions and after human activity in the room. Human activity resulted in retrieval of significantly higher concentrations of airborne spores.
Surface sampling of the carpet revealed moderate to heavy contamination despite relatively low airborne counts. Therefore, in certain situations, air sampling without concomitant surface sampling may not adequately reflect the level of microbial contamination in indoor environments. - Eduard, Wijnand, Dick Heederik, Caroline Duchaine, and Brett James Green. "Bioaerosol exposure assessment in the workplace: the past, present and recent advances." Journal of environmental monitoring 14, no. 2 (2012): 334-339.
Abstract:
Louis Pasteur described the first measurements of airborne microorganisms in 1861. A century later, the inhalation of spores from thermophilic microorganisms was shown to induce attacks of farmers' lung in patients with this disease, while endotoxins originating from Gram-negative bacteria were identified as causal agents for byssinosis in cotton workers.
Further epidemiological and toxicological studies have demonstrated inflammatory, respiratory, and pathogenic effects following exposure to bioaerosols. Exposure assessment is often confounded by the diversity of bioaerosol agents in the environment. Microorganisms represent a highly diverse group that may vary in toxicity.
Fungi and bacteria are mainly quantified as broad groups using a variety of viable and nonviable assessment methods. Endotoxins and β(1 → 3)-glucans are mainly measured by their activity in the Limulus amebocyte lysate assay, enzymes by immuno-chemical methods and mycotoxins by liquid chromatography-mass spectrometry.
Few health-based occupational exposure limits (OELs) are available for risk assessment. For endotoxins, a health-based OEL of 90 endotoxin units m−3 has been proposed in the Netherlands. A criteria document for fungal spores recently proposed a lowest observed effect level of 100 000 spores m−3 for non-pathogenic and non-mycotoxin producing species based on inflammatory respiratory effects.
Recent developments in bioaerosol assessment were presented at the Organic Dust Tromsø Symposium including molecular biological methods for infectious agents and organisms that are difficult to cultivate; studies of submicronic and hyphal fragments from fungi; the effect of biodiversity of microorganisms in asthma studies; and new/improved measurement methods for fungal antigens, enzymes and allergens.
Although exposure assessment of bioaerosol agents is complex and limited by the availability of methods and criteria, the field is rapidly evolving. - Eduard, Wijnand. "Fungal spores: a critical review of the toxicological and epidemiological evidence as a basis for occupational exposure limit setting." Critical reviews in toxicology 39, no. 10 (2009): 799-864.
Abstract
Fungal spores are ubiquitous in the environment. However, exposure levels in workplaces where mouldy materials are handled are much higher than in common indoor and outdoor environments.
Spores of all tested species induced inflammation in experimental studies. The response to mycotoxin-producing and pathogenic species was much stronger. In animal studies, nonallergic responses dominated after a single dose.
Allergic responses also occurred, especially to mycotoxin-producing and pathogenic species, and after repeated exposures.
Inhalation of a single spore dose by subjects with sick building syndrome indicated no observed effect levels of 4 × 103 Trichoderma harzianum spores/m3 and 8 × 103 Penicillium chrysogenum spores/m3 for lung function, respiratory symptoms, and inflammatory cells in the blood.
In asthmatic patients allergic to Penicillium sp. or Alternaria alternata, lowest observed effect levels (LOELs) for reduced airway conductance were 1 × 104 and 2 × 104 spores/m3, respectively.
In epidemiological studies of highly exposed working populations lung function decline, respiratory symptoms and airway inflammation began to appear at exposure levels of 105 spores/m3.
Thus, human challenge and epidemiological studies support fairly consistent LOELs of approximately 105 spores/m3 for diverse fungal species in nonsensitised populations.
Mycotoxin-producing and pathogenic species have to be detected specifically, however, because of their higher toxicity. - Haines, J., Dr. [personal communication to DJF] 11/17/2016
An American Journal of Botany article in 2011 gives a new estimate of 5.1 million fungi.
But the Dictionary of Fungi 2008 reveals that a mere 97,330 species have been described. I put that discrepancy on the NSF funding shift from taxonomy to experimental decades ago. - Haines, J., Ph.D., [Personal communication to Daniel Friedman, 2019/01/19] in " Re: Request for technical data: what % of mold genera/species can be cultured at all?'
Excerpt:
My quote of 10% petri plate viability was probably pulled out of the air (literally and figuratively). It is certainly an elusive number since we have no idea how many fungi there are. Two things come to mind as I read the new Biology of Microfungi book by De-Wei li (ed.) The first is the difference between the number fungi named on the basis of morphology and the number of fungi based on molecular data. One can assume that most if not all molds (mitosporic fungi) have an ascomycete stage.
This reduces the number of fungus species and the new changes in he code will reflect this. No longer will the telomorph and anamorph have different "legal" names and the number of legal names will reflect the number of biological species that can be tested to grow in culture (Walter Gams, Recent Changes in Fungal Nomenclature and Their Impact on Naming of Microfungi, in De-Wei Li (ed.) Biology of Microfungi, 2016).
Another issue to consider for fungal testing is the quantity of submicron articles released by molds. Cho, S-H, et al, 2005, Atmos. Environ. 39(30):5454-5465, found that S. chartarum released 514 times more submicron particles than spores. These are highly respirable and contain toxin. Think of them as fungus dandruff and more harmful to human health than spores. - Park, Ju-Hyeong, Michael Sulyok, Angela R. Lemons, Brett J. Green, and Jean M. Cox-Ganser. CHARACTERIZATION OF FUNGI IN OFFICE DUST: COMPARING RESULTS OF MICROBIAL SECONDARY METABOLITES, FUNGAL ITS REGION SEQUENCING, VIABLE CULTURE, AND OTHER MICROBIAL INDICES [PDF] (2018) U.S. Centers for Disease Control, CDC, retrieved 2020/01/08, original source: https://stacks.cdc.gov/view/cdc/60326
Abstract:
Recent developments in molecular and chemical methods have enabled the analysis of fungal DNA and secondary metabolites, often produced during fungal growth, in environmental samples.
We compared three fungal analytical methods by analyzing floor dust samples collected from an office building for fungi using viable culture, internal transcribed spacer (ITS) sequencing, and secondary metabolites using liquid chromatography-tandem mass spectrometry.
Of the 32 metabolites identified, 29 had a potential link to fungi with levels ranging from 0.04 (minimum for alternariol monomethylether) to 5,700 ng/g (maximum for neoechinulin A). The number of fungal metabolites quantified per sample ranged from eight to sixteen (average=13/sample).
We identified 216 fungal operational taxonomic units (OTUs) with the number per sample ranging from six to twenty-nine (average=18/sample).
We identified 37 fungal species using culture and the number per sample ranged from two to thirteen (average=eight/sample). Agreement in identification between ITS sequencing and culturing was weak (kappa=−0.12–0.27).
The number of cultured fungal species poorly correlated with OTUs, which did not correlate with the number of metabolites.
These suggest that using multiple measurement methods may provide an improved understanding of fungal exposures in indoor environments and that secondary metabolites may be considered as an additional source of exposure.
- Pasanen, A-L., P. Kalliokoski, P. Pasanen, M. J. Jantunen, and A. Nevalainen. "Laboratory studies on the relationship between fungal growth and atmospheric temperature and humidity." Environment International 17, no. 4 (1991): 225-228.
Abstract:
The effect of air temperature (4–30°C) and relative humidity (RH 11–96%) on the growth of two common fungi Aspergillus fumigatus and Penicillium sp. was studied in the laboratory. A short period of favorable conditions was sufficient to start fungal growth.
Temperature was not a limiting factor for fungal growth on building materials, because fungi grew at even below 10°C.
The relative humidity of air had no direct influence on the growth of fungi.
Fungi may grow at very low levels of air humidity if water is available on the surface. Thus, repeated or persistent moisture condensation or water leakage is sufficient for fungal germination and growth on building materials. - Provine, Harriet, and Susan Hadley. "Preliminary evaluation of a semisolid agar antifungal susceptibility test for yeasts and molds." Journal of clinical microbiology 38, no. 2 (2000): 537-541.
Abstract:
This report presents a semisolid agar antifungal susceptibility (SAAS) method for the rapid susceptibility screening of yeasts and molds.
The reproducibility and accuracy of the SAAS method were assessed by comparing the MICs of amphotericin B and fluconazole obtained for 10 candidate quality control (QC) American Type Culture Collection yeast strains in ≥15 replicates with those found by six independent laboratories using the National Committee for Clinical Laboratory Standards (NCCLS) M27-P broth macrodilution method (M. A. Pfaller et al., J. Clin. Microbiol. 33:1104–1107, 1995).
Overall, 96% of MICs for both drugs fell within 1 log2dilution of the modal MIC for each strain. The MICs for amphotericin B showed 99% agreement with the NCCLS proposed QC ranges within 1 log2 dilution.
Likewise, the MICs for fluconazole at ≥75% growth reduction showed 99% agreement for seven strains. Three strains, Candida albicans ATCC 24333 and ATCC 76615 and Candida tropicalis ATCC 750, showed a less sharp fluconazole endpoint at ≥75% growth reduction, but at >50% growth reduction, the agreement was 98% within 1 log2 dilution of the proposed range.
The MIC agreement within the proposed range for the suggested QC strains Candida parapsilosisATCC 22019 and Candida krusei ATCC 6258 was 100% for fluconazole and 100% within 1 log2 dilution of the proposed range for amphotericin B.
The SAAS method demonstrated the susceptibility or resistance of 25 clinical isolates of filamentous fungi such as Aspergillus fumigatus to amphotericin B, itraconazole, and fluconazole, usually within 48 h. Although the results are preliminary, this SAAS method is promising as a rapid and cost-effective screen and is worthy of concerted investigation. - Reponen, Tiina, James Lockey, David I. Bernstein, Stephen J. Vesper, Linda Levin, Gurjit K. Khurana Hershey, Shu Zheng et al. "Infant origins of childhood asthma associated with specific molds." Journal of Allergy and Clinical Immunology 130, no. 3 (2012): 639-644.
Abstract:
Background
The specific cause or causes of asthma development must be identified to prevent this disease.
Objective
Our hypothesis was that specific mold exposures are associated with childhood asthma development.
Methods
Infants were identified from birth certificates. Dust samples were collected from 289 homes when the infants were 8 months of age. Samples were analyzed for concentrations of 36 molds that comprise the Environmental Relative Moldiness Index (ERMI) and endotoxin, house dust mite, cat, dog, and cockroach allergens.
Children were evaluated at age 7 years for asthma based on reported symptoms and objective measures of lung function.
Host, environmental exposure, and home characteristics evaluated included a history of parental asthma, race, sex, upper and lower respiratory tract symptoms, season of birth, family income, cigarette smoke exposure, air conditioning, use of a dehumidifier, presence of carpeting, age of home, and visible mold at age 1 year and child's positive skin prick test response to aeroallergens and molds at age 7 years.
Results
Asthma was diagnosed in 24% of the children at age 7 years. A statistically significant increase in asthma risk at age 7 years was associated with high ERMI values in the child's home in infancy (adjusted relative risk for a 10-unit increase in ERMI value, 1.8; 95% CI, 1.5-2.2).
The summation of levels of 3 mold species, Aspergillus ochraceus, Aspergillus unguis, and Penicillium variabile, was significantly associated with asthma (adjusted relative risk, 2.2; 95% CI, 1.8-2.7).
Conclusion
In this birth cohort study exposure during infancy to 3 mold species common to water-damaged buildings was associated with childhood asthma at age 7 years. - Sayer, William J., Dudley B. Shean, and Jamshid Ghosseiri. "Estimation of airborne fungal flora by the Andersen sampler versus the gravity settling culture plate: I. Isolation frequency and numbers of colonies." Journal of Allergy 44, no. 4 (1969): 214-227.
Abstract:
The gravity settling culture (GSC) plate and the sequential impaction cascade sieve volumetric air (SICSVA) sampler devised by Andersen were critically compared in the estimation of airborne fungal flora.
One hundred twenty-seven side-by-side simultaneous 15 minute samples were obtained on Mehrlich's medium in Northern California. The SICSVA sampler collected 25 fungal types totaling 14,300 colonies, and the adjacent GSC plates displayed 18 genera in 1,091 colonies.
Statistically significant differences in total colonies and frequency of isolation were noted for Penicillium, Alternaria, Aspergillus, Gliocladium, Pullularia, Rhizopus, Saccharomyces, Stemphilium, Ustilago zeae, and other (unidentified) fungi. The capability of the SICSVA sampler to isolate volumetrically in a reproducible fashion numbers of airborne fungal spores was demonstrated.
It is suggested that these colonies can be logically grouped into a fraction that generally would lodge in the upper respiratory tract and another fraction which, because of the small spore sizes, would largely be capable of lung penetrance.
Both methods demonstrated a capacity to produce falsely negative data, but this was especially true for the GSC plate. The GSC plate method cannot be endorsed for the quantitative evaluation of airborne fungal spores. - Solomon, William R., and H. A. Burge. "Allergens and pathogens." In Indoor Air Quality, pp. 174-175. Boca Raton, Fla.: CRC Press, 1984.
- Solomon, William R. "A volumetric study of winter fungus prevalence in the air of midwestern homes." Journal of Allergy and Clinical Immunology 57, no. 1 (1976): 46-55.
Abstract:
Volumetric recoveries of airborne, mesophilic microfungi were made during winter months at three specific points in 150 single-family dwellings in southeastern Michigan. Mean levels of total isolates/m3 comprised a range of from less than 10 to over 20,000, although concurrent outdoor levels never exceeded 230/m3.
Form species of Penicillium, Aspergillus, Cladosporium, and Rhodotorula as well as non-pigmented yeasts were the types encountered most widely indoors.
Certain homes showed high recoveries of other types, including Cephalosporium, Sporobolomyces, Verticillium, and Sporothrix form species.
A positive association between indoor fungus prevalence and bedroom relative humidity was strongly suggested, and high levels were observed in well-humidified homes despite the presence of electrostatic air cleaners.
The data indicate characteristic patterns of (winter) air spora in specific homes and suggest that humidifying devices may serve as dispersion sources in addition to their permissive role in facilitating fungus growth. - Solomon, William R. "Assessing fungus prevalence in domestic interiors." Journal of allergy and clinical immunology 56, no. 3 (1975): 235-242.
Abstract:
Single-plate, Andersen sampler collections of mesophilic imperfect fungi were made at three points in and immediately outside a series of midwestern homes. During frost-free periods, emanations of dark-spored form genera predominated at both points with indoor levels averaging 25% of those in outside air.
At these times, volumetric recoveries and those by 30-min exposure of open culture plates have correlated tenuously (r = 0.29) in bedroom air of 20 homes.
During winter, form species of Penicillium, Aspergillus, Oospora, Sporothrix, yeasts, etc. predominated indoors, with levels exceeding 1,000 particles/M3 noted in over 18% of homes; outdoor concentrations never exceeded 230 particles/M3.
Comparisons of volumetric and open-plate recoveries from 50 homes during winter have revealed an almost random relationship (r = 0.06).
These findings reflect the ease with which outdoor spore clouds may penetrate structures and obscure evidence of internal fungus sources.
The data also imply that, because of size-related undersampling, open plates often seriously misrepresent prevalence levels and occasionally can exclude abundant types from recovery.
The fungus flora of enclosed spaces merits further critical study by volumetric techniques of calculable efficiency in a setting that minimizes contamination from without. - U.S. CDC, BASIC FACTS [ABOUT MOLD] [PDF], Centers for Disease Control and Prevention,
retrieved 2018/05/17, original source: https://www.cdc.gov/mold/faqs.htm#test
Excerpt:
Generally, it is not necessary to identify the species of mold growing in a residence, and CDC does not recommend routine sampling for molds.
Current evidence indicates that allergies are the type of diseases most often associated with molds.
Since the susceptibility of individuals can vary greatly either because of the amount or type of mold, sampling and culturing are not reliable in determining your health risk.
If you are susceptible to mold and mold is seen or smelled, there is a potential health risk; therefore, no matter what type of mold is present, you should arrange for its removal.
Furthermore, reliable sampling for mold can be expensive, and standards for judging what is and what is not an acceptable or tolerable quantity of mold have not been established. - Centers for Disease Control and Prevention and U.S. Department of Housing and Urban Development. HEALTHY HOUSING REFERENCE MANUAL [PDF] Atlanta: US Department of Health and Human Services; 2006. retrieved 2018/05/17, original source: U.S. Centers for Disease Control and Prevention, https://www.cdc.gov/nceh/publications/books/housing/housing_ref_manual_2012.pdf
- Vesper, Stephen J., Wing Wong, C. Mike Kuo, and Duane L. Pierson. "Mold species in dust from the International Space Station identified and quantified by mold-specific quantitative PCR." Research in microbiology 159, no. 6 (2008): 432-435.
Abstract:
Dust was collected over a period of several weeks in 2007 from HEPA filters in the U.S. Laboratory Module of the International Space Station (ISS).
The dust was returned on the Space Shuttle Atlantis, mixed, sieved and the DNA was extracted. Using a DNA-based method called mold-specific quantitative PCR (MSQPCR), 39 molds were measured in the dust.
Potential opportunistic pathogens Aspergillus flavus and Aspergillus niger and potential moderate toxin producers Penicillium chrysogenum and Penicillium brevicompactum were noteworthy.
No cells of the potential opportunistic pathogens Aspergillus fumigatus, Aspergillus terreus, Fusarium solani or Candida albicans were detected.
Question: Where Can I Buy Mold Test Kits for do-it-yourself testing?
I will be calling one of the testing inspectors that you list
at MOLD & ENVIRONMENTAL INSPECTORS .
In addition, is there any device I can purchase which would allow me to make an independent test? - Thanks J.O.
Reply:
Do-it-yourself mold tests are widely available at hardware stores and building suppliers as well as online. The tests usually fall into one of two groups:
Adhesive tape collection surface particles: particle or surface debris or suspected mold on a surface - see https://inspectapedia.com/sickhouse/Adhesive_Tape_Particle_Test.php for detailed procedures on how this test sort is used (to avoid any conflict of interest, or even an apparent conflict, don't sent your sample to us)
Mold culture kits: a plastic petri dish of growth medium is exposed to air, re-sealed, and you or the lab gets to see what grows. Since 90- 95% of molds won't grow on any culture, this is an unreliable way to screen a building for problem mold, though cultures do have widespread and valid use in the lab.
Even in a test or research lab where there are reasons to culture mold the technician needs to select the proper mold culture media that is likely to support growth by the particular classof molds under study.
MEA or Malt Extract Agar is a common culture medium and PDA or Potato Dextrose Agar is another that will grow quite a few but nowhere near all fungii.
But to try to use cultures to grow a mold sample that's been collected by some means, lab experts would typically use a whole battery of various culture media with or without certain mold inhibitors (to control rapidly-growing mold from over-growing other molds in the sample, thus obscuring their presence).
MOLD CULTURE TEST KIT VALIDITY has details.
Airborne tests for mold are not usually performed by a building owner as special equipment is needed.
See ACCURACY OF AIR TESTS for MOLD and for more detail,
AIRBORNE PARTICLE TEST SAMPLING CASSETTE STUDY
and AIRBORNE MOLD SPORE COUNT ACCURACY.
And frankly, for serious mold or water damage investigation cases, using any of these "mold test" methods to produce useful results needs to be combined with an expert building inspection and case history as well as occupant interview.
At ACCURACY OF AIR TESTS for MOLD we provide details about the accuracy of various mold test methods.
The reliance on mold "tests" without a thorough, expert onsite inspection, history taking, etc. is simply unjustified. And that may be why whomever you paid to do these tests can't be more helpful to you.
Properly, and providing an onsite investigation was justified in the first place then the person who inspected the site, took its history, understands the building, its occupants, its environment, should be able to make a meaningful interpretation of various tests done in the building that supplement the more thorough site investigation.
See MOLD / ENVIRONMENTAL EXPERT, HIRE ?- when to hire a mold expert.
Relying on a mold "test" is profitable for the test company and lab but by itself, can be misleading, especially where low numbers or low mold-level findings were the results found in the test. Even high mold level findings can be misleading as the test may not have detected the most problematic mold present.
All of that said, you can hire someone to read and interpret and discuss your report, and then to suggest further investigative steps that could be helpful.
See MOLD & ENVIRONMENTAL INSPECTORS.
Bottom line: in our experience and opinion, relying on mold tests alone to diagnose a building is a risky proposition.
Special thanks to Dr. John Haines for discussing mold testing & indoor mold frequencies. - Ed. 2016/11/26
References on Use of Cultures to Grow or Study Mold
- Atlas of Clinical Fungi, 2nd Ed., GS deHoog, J Guarro, J Gene, & MJ Figueras, Centraalbureau voor Schimmelcultures, Universitat Rovira I Virgili, 2000, ISBN 90-70351-43-9
- Behrens, Otto K., and Joseph W. Corse. "Process and culture media for producing penicillin." U.S. Patent 2,449,195, issued September 14, 1948.
Excerpt:
By the practice of this invention the yield of penicillin has been substantially increased. Thus under comparative conditions the yield of penicillin has been increased from 50 to upwards of percent. This range is of course illustrative and lesser or greater yields are within the contemplation of the invention depending upon the conditions under which the penicillin'is produced. - Brancato, F. P., and N. S. Golding. "The diameter of the mold colony as a reliable measure of growth." Mycologia 45, no. 6 (1953): 848-864.
- Etchells, J. L., R. N. Costilow, T. A. Bell, and A. L. Demain. "Control of molds during the enumeration and isolation of yeasts from soil and plant material." Applied microbiology 2, no. 5 (1954): 296.
Excerpt: In a large percentage of the samples no yeast colonies grew - Fifth Kingdom, Bryce Kendrick, ISBN13: 9781585100224, - we recommend the CD-ROM version of this book.
This 3rd/edition is a compact but comprehensive encyclopedia of all things mycological. Every aspect of the fungi, from aflatoxin to zppspores, with an accessible blend of verve and wit. The 24 chapters are filled with up-to-date information of classification, yeast, lichens, spore dispersal, allergies, ecology, genetics, plant pathology, predatory fungi, biological control, mutualistic symbioses with animals and plants, fungi as food, food spoilage and mycotoxins. - Fungi, Identifying Filamentous, A Clinical Laboratory Handbook, Guy St-Germain, Richard Summerbell, Star Publishing, 1996, ISBN 0-89863-177-7 (English)
- Funnell-Harris, Deanna L., Louis K. Prom, and Jeffrey F. Pedersen. "Isolation and characterization of the grain mold fungi Cochliobolus and Alternaria spp. from sorghum using semiselective media and DNA sequence analyses." Canadian journal of microbiology 59, no. 2 (2012): 87-96.
Excerpt: Thirty-one percent of colonies transferred from the semiselective media did not grow ... - Graham, D. C., K. H. Steinkraus, and L. Ross Hackler. "Factors affecting production of mold mycelium and protein in synthetic media." Appl. Environ. Microbiol. 32, no. 3 (1976): 381-387.
- Greene, H. C., and E. B. Fred. "Maintenance of vigorous mold stock cultures." Industrial & Engineering Chemistry 26, no. 12 (1934): 1297-1299.
- Hodges, F. Allen, James R. Zust, Howard R. Smith, A. A. Nelson, B. H. Armbrecht, and A. D. Campbell. "Mycotoxins: aflatoxin isolated from Penicillium puberulum." Science 145, no. 3639 (1964): 1439-1439.
Excerpt: One of the mold cultures was initially recognized as a Penicillium species - Jang, Hung-Der, Yuh-Yih Lin, and Shang-Shyng Yang. "Effect of culture media and conditions on polyunsaturated fatty acids production by Mortierella alpina." Bioresource technology 96, no. 15 (2005): 1633-1644.
- Kozak Jr, Peter P., and Janet M. Gallup. "Method for obtaining mold spore material." U.S. Patent 4,280,000, issued July 21, 1981.
- Meletiadis, Joseph, Jacques FGM Meis, Johan W. Mouton, and Paul E. Verweij. Analysis of growth characteristics of filamentous fungi in different nutrient media [PDF] Journal of Clinical microbiology 39, no. 2 (2001): 478-484.
Excerpt:
None of the media supported the growth of S. prolificans sufficiently. Apparent were other effects of the media, such as the delay of germination of spores and conidia as well as the lower elongation rates of hyphae of all species in YNB medium, processes which were enhanced in AM3. By contrast with the growth of yeasts, where the stationary phase is reached within 30 h (15, 36), the growth of filamentous fungi is characterized by smoother curves and long transition periods although it depended on the medium and species.
- Mycology, Fundamentals of Diagnostic [Book sold at Amazon] Fran Fisher, Norma B. Cook, W.B. Saunders Co. 1998, ISBN 0-7216-5006-6
- Nielsen, Kristian Fog, G. Holm, L. P. Uttrup, and P. A. Nielsen. "Mould growth on building materials under low water activities. Influence of humidity and temperature on fungal growth and secondary metabolism." International Biodeterioration & Biodegradation 54, no. 4 (2004): 325-336.
- Provine, Harriet, and Susan Hadley. "Preliminary evaluation of a semisolid agar antifungal susceptibility test for yeasts and molds." Journal of clinical microbiology 38, no. 2 (2000): 537-541.
- Reich, M., P. P. Bosshard, M. Stark, K. Beyser, and S. Borgmann. Species identification of bacteria and fungi from solid and liquid culture media by MALDI-TOF mass spectrometry [PDF] J. Bacteriol. Parasitol 10 (2013): 2155-9597.
- Tournas, V., M. E. Stack, P. B. Mislivec, H. A. Koch, and R. Bandler. "Chapter 18, Yeasts, molds and mycotoxins." Bacteriological analytical manual (BAM). Food and Drug Administration, Silver Spring, MD. https://www. fda. gov/food/foodscienceresearch/laboratorymethods/ucm2006949. htm (2001).
- Also seeReferences or Citations below
...
Reader Comments, Questions & Answers About The Article Above
Below you will find questions and answers previously posted on this page at its page bottom reader comment box.
Reader Q&A - also see RECOMMENDED ARTICLES & FAQs
On 2023-08-21 by InspectApedia Publisher
@John Spoerer,
I am sorry that you wasted money on a "mold test kit" using a culture plate. Roughly 90% of molds won't grow in any culture media at all, so the kit is 90% "wrong" the moment you take off the lid.
Furthermore, the molds that do grow are ones that like the culture media, which does not mean that they're the most significant indoor molds present.
And finally (well it's never the last word), no, we can't identify the mold genera/species from just the appearance of a culture. You'd need to examine the culture with a microscope.
All of that griping aside - if your home has had leaks and water has run in ceilings and walls, especially if those are made using drywall, there is a significant risk that there is mold growth in those cavities as well as in insulation that got wet.
The bottom line of what's needed is to follow the water, find the mold, remove it, and fix the cause.
You report that the roof has been repaired, so what remains is to find and remove the mold, clean the exposed surfaces, then finish the repair.
For older people who may be at more risk - as you are on oxygen - I would take the mold hazard very seriously. The risk is that SOME molds (certainly not all of them) produce small, harmful mold spores that can produce a hard-to-cure fungal lung infection.
Start by asking your doctor for advice and follow that.
Do not let some madman run through the home chopping ceilings and walls willy-nilly, not just because of un-needed cost, but because it could significantly increase the health risk to you both.
What IS appropriate, in my opinion, is a thorough on-site inspection by an experienced mold expert who will look at the history of leaks, of where water went, and the building materials involved.
Based on that work s/he should identify the areas of greatest risk of hidden mold and should investigate those building cavities - often a simple small test cut - a few square inches - is enough to look into the cavity, at the nearby wood framing, insulation, and to examine the back or hidden surfaced of the drywall.
If mold is found there, more-extensive demolition and cleaning will be needed.
I apologize, because I know this is much easier for me to say than for you to do (and afford) but IF significant demolition and cleaning are needed in your rental home, my guess is that your doctor will say you should not remain in the building during that work - again because of health risks to you both.
Please keep me posted on how this progresses, and don't hesitate to ask follow-up questions as needed.
Daniel Friedman
On 2023-08-21 by John Spoerer
My wife and I are senior disabled on social security so funds are very limited. We have lived in this rental house for 12 years and it had a roof leak water through the bathroom walls for years before the roof was repaired.
The drywall would get soft. Anyway, we are both on oxygen now and have breathing problems. I got a mold test kit and followed the directions to a T in the bathroom. Exposed for one hour in a Petrie dish.
Wondering if it is a serious type of mold or not. Thank you for your time.
This reader's Q&A were originally posted at HOW TO CONTACT InspectApedia.com
...
Continue reading at MOLD CULTURE SAMPLING METHOD or select a topic from the closely-related articles below, or see the complete ARTICLE INDEX.
Or see these
Recommended Articles
- AIRBORNE PARTICLE SIZE TABLE
- MOLD CULTURE SAMPLING METHOD
- MOLD CULTURE PHOTOS
- MOLD CULTURE TEST ERRORS
- MOLD CULTURE TEST KIT VALIDITY The use, accuracy, and reliability of mold culture test kits for screening buildings for mold contamination
Suggested citation for this web page
MOLD CULTURE TEST KIT VALIDITY at InspectApedia.com - online encyclopedia of building & environmental inspection, testing, diagnosis, repair, & problem prevention advice.
Or see this
INDEX to RELATED ARTICLES: ARTICLE INDEX to MOLD CONTAMINATION & REMEDIATION
Or use the SEARCH BOX found below to Ask a Question or Search InspectApedia
Ask a Question or Search InspectApedia
Questions & answers or comments about buying & using do-it-yourself mold test kits based on culture plates..
Try the search box just below, or if you prefer, post a question or comment in the Comments box below and we will respond promptly.
Search the InspectApedia website
Note: appearance of your Comment below may be delayed: if your comment contains an image, photograph, web link, or text that looks to the software as if it might be a web link, your posting will appear after it has been approved by a moderator. Apologies for the delay.
Only one image can be added per comment but you can post as many comments, and therefore images, as you like.
You will not receive a notification when a response to your question has been posted.
Please bookmark this page to make it easy for you to check back for our response.
IF above you see "Comment Form is loading comments..." then COMMENT BOX - countable.ca / bawkbox.com IS NOT WORKING.
In any case you are welcome to send an email directly to us at InspectApedia.com at editor@inspectApedia.com
We'll reply to you directly. Please help us help you by noting, in your email, the URL of the InspectApedia page where you wanted to comment.
Citations & References
In addition to any citations in the article above, a full list is available on request.
- In addition to citations & references found in this article, see the research citations given at the end of the related articles found at our suggested
CONTINUE READING or RECOMMENDED ARTICLES.
- Carson, Dunlop & Associates Ltd., 120 Carlton Street Suite 407, Toronto ON M5A 4K2. Tel: (416) 964-9415 1-800-268-7070 Email: info@carsondunlop.com. Alan Carson is a past president of ASHI, the American Society of Home Inspectors.
Thanks to Alan Carson and Bob Dunlop, for permission for InspectAPedia to use text excerpts from The HOME REFERENCE BOOK - the Encyclopedia of Homes and to use illustrations from The ILLUSTRATED HOME .
Carson Dunlop Associates provides extensive home inspection education and report writing material. In gratitude we provide links to tsome Carson Dunlop Associates products and services.

