Formaldehyde Gas HCHO Exposure Limits
Formaldehyde Gas HCHO Exposure Limits
Formaldehyde Gas (Formalin Gas) Exposure Standards & Limits in Buildings
- POST a QUESTION or COMMENT about how to test for indoor formaldehyde gas contaminants
Formaldehyde Gas Exposure Limits in Buildings:
What are the allowable exposure limits & standards for formaldehyde gas? What levels of formaldehyde gas are acceptable. Formaldehyde exposure and/or testing standards are presented for Australia, Canada, the E.U., the U.S., the E.U., New Zealand
Page top photo, the author's GasTech™ pump using a colorimetric gas detection tube in a private home.
InspectAPedia tolerates no conflicts of interest. We have no relationship with advertisers, products, or services discussed at this website.
- Daniel Friedman, Publisher/Editor/Author - See WHO ARE WE?
Formaldehyde Gas HCHO Characteristics, Toxicity, Gas Exposure Limits
Formaldehyde is an organic chemical with formula HCHO, CAS No. 50-00-0 that is very widely used in industry as well as in a plethora of consumer products from cosmetics and clothing to furnishings and construction materials as well as in disinfectants and preservatives.
Formaldehyde is an intermediate chemical used in the production of resins (e.g. glues in fiberboard and OSB, and in wrinkle-resistant clothing), industrial chemicals, preservatives (including in some cosmetics and skin creams), and in shampoos and glues. Formaldehyde may also be present in food or may be generated by smoking indoors.
In the U.S. formaldehyde is detected in outdoor (ambient) air in urban areas at levels between 11 and 20 parts per billion (ppb) while indoors formaldehyde levels are commonly reported between 0.10 and 3.68 parts per million (ppm). - US EPA (2000)
There are currently no national standards in place for formaldehyde in composite wood products. However, EPA is in the process of finalizing rules that will set limits on formaldehyde emissions from composite wood products such as hardwood plywood, medium-density fiberboard, and particle board that are sold, supplied, offered for sale, manufactured, or imported in the United States. - U.S. EPA cited below
Article Contents
Human Response to Various Levels of Formaldehyde Concentration in Building Air
Formaldehyde: (or Formalin in some literature) gas exposure limits - As an additional example using Formaldehyde, in a screen we may test for very low levels in a building (.05 ppm), but the range of human response (also widely variable individually) may be summed as follows:
Health Effects of Exposure to Formaldehyde1 |
||
| Formaldehyde Level in Air | Expected Health Effects | Comments |
| > .01 ppm | mild irritation or allergic sensitization in some people | [>0.0123 mg/M3] |
| > 0.5 ppm | irritation to eyes & mucous membranes | [>0.615 mg/M3] |
| > 1.0 ppm | possible nasopharyngeal cancer | [>1.23 mg/M3] |
| 3.0 ppm | respiratory impairment and damage | [ 3.684 mg/M3 ] |
| Approx. 0.0001 mg/m3 | Cancer risk level of 1 in 1-million | Amoore (1983) [0.0008 ppm] |
| 0.004 mg/m3 | ATSDR chronic MRL | ATSDR (1997) [0.32572 ppm] |
| 203 mg/m3 | LC-50: Lethal concentration for rats | US DEHS (1993) [165.3 ppm] |
| 400 mg/m3 | LC-50: Lethal concentration for mice | US DEHS (1993) [325.72 ppm] |
Notes to the table above
1. The values in this table are commonly-cited health effects of exposure to formaldehyde in air, not the legislated / recommended exposure limits nor exposure recommendations for formaldehyde. Formaldehyde exposure limits or recommendations are given below.
2. Watch out: mg/m3 (micrograms per cubic meter) cannot be equated to ppm (parts per million) or ppb (parts per million or parts per billion) concentrations of a substance in air. Conversion between these measurement scales depends on the molecular weight of the substance as well as on the temperature and pressure in the environment being measured.
3. Details about converting concentrations of formaldehyde between mg/m3 and ppm and example calculations converting levels of formaldehyde between ppm and mg/m3 are found at CONVERT PPM - mg/m3 Bracketed numbers in the table above are calculated as shown at CONVERT PPM - mg/m3 assuming conditions of sea level pressure (1 ATM) and assuming a temperature of 25°C.
See FORMALDEHYDE HAZARDS for a table of "Common Concentrations of Formaldehyde (HCHO) in Indoor & Outdoor Air " - formaldehyde levels found outdoors and in buildings and a comparison of those levels with current residential exposure standards for formaldehyde.
Formaldehyde MSDS sheets: This FORMALDEHYDE MSDS [PDF] from Mallinckrodt Chemicals notes that typical composition of formaldehyde in industrial use is comprised of 37% formaldehyde, 10-15% methyl alcohol, and the remainder water. Synonyms for formaldehyde exposure are Formaldehyde 37%, Formalin, Morbicid Acid, Methylene Oxide, Methyl aldehyde, all bearing the CAS No.: 50-00-0 and expressed by the chemical formula HCHO and CH3OH in water.
Common industrial or workplace formaldehyde gas exposure limits or Recommendations
Formaldehyde is normally present at low levels in buildings, usually below 0.03 ppm both indoors and outdoors. However, buildings with high levels of pressed wood products can have higher indoor levels. For example, many manufactured homes have levels well above 0.03 ppm, due to their relatively small volume and large surface area of formaldehyde emitting materials.
U.S. Indoor Formaldehyde Exposure Limits, Recommendations, Regulatory Advisory Numbers
There are generally two different categories of exposure limits for formaldehyde (and other chemicals or contaminants in the workplace):
- Short-time exposure levels are used for preventing acute health effects of formaldehyde on individuals
- Long-term exposure levels are used for preventing the chronic health effects of formaldehyde. "Long term" exposure values are typically given as time weighted average (TWA) values for 8 or 24-hour time periods and are intended to protect people in the indoor environment from exposure to formaldehyde over a lifetime. (Salthammer et als., 2010).
- ACGIH, American Conference of Industrial Hygienists
(www.acgih.org)
- ACGIH STEL for Formaldehyde: 0.4 mg/m3
- TLV-C proposed by the American Conference of Governmental Industrial Hygienists (ACGIH) is 0.3 ppm.
- AIHA, American Association of Industrial Hygiene, EPRG Emergency Response Planning Guidelines (www.aiha.org)
- AIHA EPRG-1 for Formaldehyde: 1.2 mg/m3 (also given as 1 ppm)
- AIHA EPRG-2 for Formaldehyde: 2 mg/m3
Watch out: The AIHA's EPRG (Emergency Response Planning Guide) for formaldehyde can be misleading when considering consumer or building occupant concerns about possible formaldehyde outgassing from laminated flooring. The ERPG-1 of roughly 1 ppm is only for up to an hour exposure and allows for mild, transient adverse health effects.
- California Formaldehyde Regulations:
Permissible Formaldehyde Exposure Level (PEL)
- Employee exposure. Employee exposure means the exposure to airborne formaldehyde which would occur without corrections for protection provided by any respirator that is in use.
- Formaldehyde. Formaldehyde means the chemical substance, HCHO, Chemical Abstracts Service Registry No. 50-00-0.
- (c) Permissible Exposure Limit (PEL)
(1) Time Weighted Average (TWA): The employer shall assure that no employee is exposed to a concentration of airborne formaldehyde which exceeds 0.75 parts formaldehyde per million parts of air (0.75 ppm) as an 8-hour TWA.
(2) Short Term Exposure Limit (STEL): The employer shall assure that no employee is exposed to a concentration of airborne formaldehyde which exceeds two parts formaldehyde per million parts of air (2 ppm) as a 15 minute STEL.
Source: Title 8. Industrial Relations Subchapter 7. General Industry Safety Orders Group 16. Control of Hazardous Substances Article 110. Regulated Carcinogens, §5217. Formaldehyde, - retrieved 11 March 2015, original source: https://www.dir.ca.gov/title8/5217.html - Title 8. Industrial Relations Subchapter 7. General Industry Safety Orders Group 16.
- California's Air Resources Board (CARB) Comments on Composite Wood Products ATCM and Formaldehyde: [Excerpt]
- Formaldehyde is produced on a large scale worldwide. One major use includes the production of wood binding adhesives and resins. The Air Resources Board (ARB) evaluated formaldehyde exposure in California and found that one of the major sources of exposure is from inhalation of formaldehyde emitted from composite wood products containing urea-formaldehyde resins. The International Agency for Research on Cancer (IARC) reclassified formaldehyde from "probably carcinogenic to humans" to "carcinogenic to humans" in 2004, based on the increased risk of nasopharyngeal cancer. Formaldehyde was also designated as a toxic air contaminant (TAC) in California in 1992 with no safe level of exposure. State law requires ARB to take action to reduce human exposure to all TACs.
- The Composite Wood Products Regulation is a California Air Resources
Board (CARB) regulation that reduces public exposure to formaldehyde
through the establishment of strict emission performance standards
on particleboard, medium density fiberboard and hardwood plywood
(collectively known as composite wood products). The regulation, adopted
in 2007, established two phases of emissions standards: an initial
Phase I, and later, a more stringent Phase 2 that requires all finished
goods, such as flooring, destined for sale or use in California to be made
using complying composite wood products. As of January, 2014 only
Phase 2 products are legal for sale in California.
- "CARB Consumer Factsheet", Californian Environmental Protection Agency Air Resources Board (CARB), 03/03/2015, http://www.arb.ca.gov/html/fact_sheets/composite_wood_flooring_faq.pdf - "Composite Wood Products ATCM", [California regulations on composite wood products and off gassing: ] California Environmental Protection Agency, California Air Resources Board (CARB), 3 March 2015, original source: http://www.arb.ca.gov/toxics/compwood/compwood.htm
- HUD standards limit the formaldehyde emissions of materials used in manufactured housing to bring the ambient level to below 0.40 ppm - four times the 0.10 ppm limit recommended by most health and standards organizations, including ASHRAE and ANSI.
- NIOSH REL:
Recommended Exposure Limits for Formaldehyde:
- NIOSH REL for formaldehyde: 0.02 mg/m3
- NIOSH IDLH for Formaldehyde: 25 mg/m3
- Ca TWA 0.016 ppm C 0.1 ppm [15-minute];
- NIOSH STEL 0.1 ppm
- NIOS recommended limit for occupation al exposure 0.016 ppm
- OSHA PEL:
Permissible Exposure Limits for Formaldehyde
- OSHA PEL for formaldehyde: 0.9 mg/m3
- TWA 0.75 ppm ST 2 ppm
- OSHA Standard Number 1910.1048: FORMALDEHYDE EXPOSURE LIMITS [PDF]
- The Occupational Safety and Health Administration (OSHA) has set the STEL for formaldehyde at 2 ppm in 15 min and the permissible exposure limit time-weighted average (PEL-TWA) at 0.75 ppm.
- U.S. Consumer Product Safety Commission
UPDATE on FORMALDEHYDE [PDF]: US CPSC, retrieved Feb 2013, renewed 17 April 2015, original source:
www.cpsc.gov/PageFiles/121919/AN%20UPDATE%20ON%20FORMALDEHYDE%20final%200113.pdf - U.S. State Labor Department example of Formaldehyde Regulations:
FORMALDEHYDE BASIC RULES [PDF] Washington State Department of Labor and Industries, retrieved 12/13/2010, original source: http://www.lni.wa.gov/wisha/rules/formaldehyde/html/296-856-200.htm - U.S. Environmental Protection Agency:
- US EPA: Formaldehyde Hazard Summary [PDF] retrieved 17 April 2015, original source: http://www.epa.gov/airtoxics/hlthef/formalde.html
- US EPA: Questions and Answers Regarding Laminate Flooring [PDF] retrieved 13 April 2015, original source: http://www2.epa.gov/formaldehyde/questions-and-answers-regarding-laminate-flooring-0
- US EPA: Protect Against Exposure to Formaldehyde [PDF] retrieved 12 April 2015, original source: http://www2.epa.gov/formaldehyde/protect-against-exposure-formaldehyde
- US EPA Proposed Formaldehyde Rules:
The U.S. EPA has been considering adopting the California formaldehyde and composite wood products rules since 2009, and a proposed rule was issued in 2013.
This proposed EPA rule includes testing requirements, laminated product provisions, product labeling requirements, chain of custody documentation requirements, a stockpiling prohibition and enforcement provisions. The proposed rule also recommends a third-party certification framework, including requirements and responsibilities for third-party certifiers.
Quoting from US EPA "Formaldehyde Emission Standards for Composite Wood Products", found at http://www2.epa.gov/formaldehyde
Proposed Rules to Implement the Formaldehyde Standards for Composite Wood Products Act
There are currently no national standards in place for formaldehyde in composite wood products. However, EPA is in the process of finalizing rules that will set limits on formaldehyde emissions from composite wood products such as hardwood plywood, medium-density fiberboard, and particleboard that are sold, supplied, offered for sale, manufactured, or imported in the United States.
Congress tasked EPA with developing regulations to implement the Formaldehyde Standards for Composite Wood Products Act, or Title VI of the Toxic Substances Control Act (TSCA). On June 10, 2013, EPA proposed two regulations:
The first proposal would establish a framework for a third-party certification program to ensure that composite wood panel producers comply with the formaldehyde emission limits established under TSCA Title VI. We refer to this proposed regulation as the Third-Party Certification Program Framework Rule.
The second proposal would implement formaldehyde emission standards under Title VI of TSCA and would apply to hardwood plywood, medium-density fiberboard, particleboard, and finished goods containing these products that are sold, supplied, offered for sale, or manufactured (including imported) in the United States. We refer to this regulation as the Implementation Rule.
In an ongoing effort to reach out to potentially affected stakeholders, EPA met and continues to meet with companies and trade associations that represent, among other members, producers of laminated products. As part of this effort, EPA specifically requested data on formaldehyde emissions from laminated products, as well as comments and information on the proposed definition of laminated products. The comment period for these proposed rules is now closed and EPA anticipates publishing a combined final rule by the end of 2015.
Also see
U.S. Environmental Protection Agency. Health and Environmental Effects Profile for Formaldehyde. EPA/600/x-85/362. Environmental Criteria and Assessment Office, Office of Health and Environmental Assessment, Office of Research and Development, Cincinnati, OH. 1988.
- Formaldehyde Standard for Composite Wood Products Act, or Title VI of the Toxic Substances Control Act, U.S. Congressional legislation, 2010. This act established emission standards for formaldehyde from composite wood products and directed the U.S. Environmental Protection Agency to propose rules to enforce the Act’s provisions.
- See detailed citations atReferences or Citations
Definitions of Common Exposure Limit Acronyms: EPERG, STEL, LC50, IDLH, REL, STEL, PEL
The following list of definitions of common exposure limit recommendations is excerpted from US EPA: Formaldehyde Hazard Summary. Notice that these are generally industrial or workplace environment measures.
- AIHA ERPG - American Industrial Hygiene Association's Emergency Response Planning Guidelines.
- ERPG 1 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed up to one hour without experiencing other than mild transient adverse health effects or perceiving a clearly defined objectionable odor;
- ERPG 2 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed up to one hour without experiencing or developing irreversible or other serious health effects that could impair their abilities to take protective action.
- ACGIH STEL - American Conference of Governmental and Industrial Hygienists' Short-Term Exposure Limit expressed as a time-weighted average exposure; the concentration of a substance which should not be exceeded at any time during a workday.
- LC50 (Lethal Concentration50) - A calculated concentration of a chemical in air to which exposure for a specific length of time is expected to cause death in 50% of a defined experimental animal population.
- NIOSH IDLH - National Institute of Occupational Safety and Health's Immediately Dangerous to Life or Health limit; NIOSH recommended exposure limit to ensure that a worker can escape from an exposure condition that is likely to cause death or immediate or delayed permanent adverse health effects or prevent escape from the environment.
- NIOSH REL - NIOSH's Recommended Exposure Limit; NIOSH recommended exposure limit for an 8- or 10-h time-weighted average exposure and/or ceiling.
- OSHA PEL - Occupational Safety and Health Administration's Permissible Exposure Limit expressed as a time-weighted average; the concentration of a substance to which most workers can be exposed without adverse effect averaged over a normal 8-h workday or a 40-h workweek.
- OEHHA REL - Reference Exposure Levels: RELs are intended to protect the most-sensitive individuals and include the provision of a safety margin.
Formaldehyde HCHO Limits / Recommendations in the European Union E.U.
In the E.U. formaldehyde use in household products and chemical outgassing from wood products has been more closely regulated since 2003 (Germany) or 2006 (elsewhere), and as The Times pointed out, in Japan it is home builders who are required to limit the overall formaldehyde levels in new construction. - op. cit. 3/11
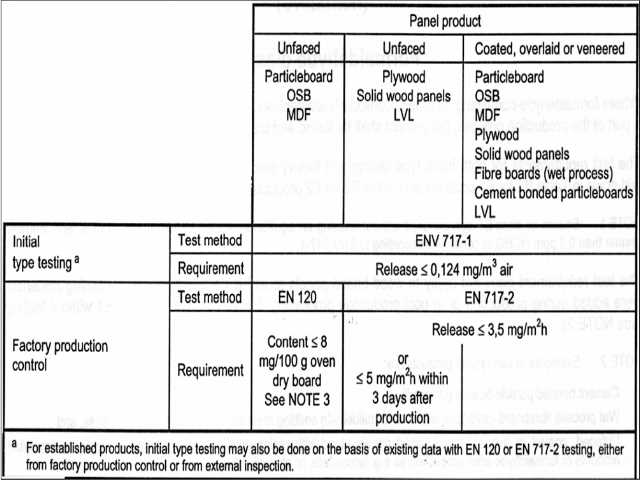
Formaldehyde release from products is regulated in the E.U. for panel products such as un-faced cement-bonded particleboards, wet-processed un-faced fibreboard (when no formaldehyde emitting resin was added to the process) and for un-faced, coated or overlaid wood based panels (which might include laminate flooring) when glued with resins that emit either no formaldehyde or only negligible amounts of formaldehyde after production. The authors (Schwab et als) cite the use of isocyanate or phenolic glues as examples.
There are two formaldehyde outgassing level certification indicators used on products in the E.U.
- Germany, through the German Association of Producers of Fabricated Houses (BDF), specifies 0.03 ppm panel boards asserted as equivalent to Japan's formaldehyde emission class F
- "Blue Angel" labels indicate that the board has a formaldehyde outgas rate of 0.05 ppm (with changes or diminution over what lifetime is not stated)
In Europe there is one reference method and there are three or four derived methods for testing the level of formaldehyde in a building or in a material:
Reference Formaldehyde Testing Method: The E.U. Formaldehyde Testing Chamber method EN 717-1 with three different volume options.
The chamber method used in the E.U. involves a large-enough chamber that it can contain multiple structures of cabinetry or shelving or sheet goods. This method is illustrated and discussed by Schwab et als. (un-dated). Tested components are sealed in the test chamber for up to 28 days, permitting formaldehyde emission level measurements in ppm or mg/m3.
Derived Formaldehyde Testing Methods in the E.U.:
- Dessicator method for formaldehyde testing # ISO/DIS 12460-4 or JISD A 1460 / JAS 233 (possibly). This testing method uses a dessicator in a container of material and is used for testing the formaldehyde release from both coated or un-coated particleboard or medium density fiberboard, but using quite small physical samples.
- Flask method for formaldehyde testing # EN 717-3. This method determines the level of release of formaldehyde in mg/kg and according to Schwab et als is suitable only for internal production control of wood based panels by fabricators, presumably because there is no published official limit value.
- Gas Analysis method for formaldehyde testing # EN 717-2. This approach is used for coated particle board, for either coated or un-coated plywood, and determines the formaldehyde content in mg/M2 xh
- Perforator method for formaldehyde testing # EN 120 permits determination of formaldehyde content in mg/100 g of substance using a toluene extraction method. This approach is used with particleboard (PB), medium density fiberboard (MDF), and oriented strand board (OSB).
Formaldehyde Emission Limits for the E.U. were published by Schwab et als (cited below) and vary depending on the testing method used. The authors we cite provide a helpful chart from which we excerpt and adapt below. Please see the original for complete details.
[Click to enlarge any image]
- Using EN 120 for un faced particleboard, OSB or MDF, the formaldehyde content is limited to <= 8mg/100g of oven-dried board with moisture content specification depending on the panel or board type.
Watch out: moisture content is a key factor in testing these products against formaldehyde emission standards, and if the moisture content is not within specification the test results will not be valid. - Using ENV 717-1 for initial type testing of established products the limit is a formaldehyde release of <= 0.124 mg/m3 of air for all types of panel or board products including particleboard, plywood, OSB, MDF, solid wood, etc.
- Using ENV 717-2 the limit depends on whether the tested material is faced or coated or not
Australia & New Zealand Formaldehyde HCHO Emission Standards, Limits & Regulations
- NICNAS Priority Existing Chemical Assessment Report No. 28 – Formaldehyde. Sydney, NICNAS (2006), available at: www.nicnas.gov.au
- AS/NZS 1859.1:2004: Reconstituted wood-based panels – Specifications – Particleboard
- AS/NZS 1859.2:2004: Reconstituted wood-based panels – Specifications – Dry-processed Fibreboard
- AS/NZS 1860.1:2002: Particleboard flooring – Specifications
- AS/NZS2269.0: 2008: Plywood – Structural Part 0 Specifications1
- AS/NZS 2270:2006: Plywood and blockboard for interior use1
- AS/NZS 2271:2004: Plywood and blockboard for exterior use1
- AS/NZS 2272: 2006: Plywood-Marine1
- See http://www.ewp.asn.au/library/standards.html for direct links to these standards
Australian Standards, labelling and product certification, are available at Engineered Wood Products Association of Australasia website at: www.paa.asn.au
Australian, E.U. & U.S. Formaldehyde Emission Standards & Testing References:
- Australian Competition & Consumer Commission, "Product Safety in Australia, Formaldehyde in Consumer Products", retrieved 29 March 2015, original source: https://www.productsafety.gov.au/content/index.phtml/itemId/973697
- Australian Government Department of Health, "Formaldehyde in Pressed Wood Products Safety Fact Sheet, CAS No. 50-00-0", National Industrial Chemicals Notification and Assessment Scheme, retrieved 29 March 2015, original source: http://www.nicnas.gov.au/communications/
publications/information-sheets/existing-chemical-info-sheets/formaldehyde-in-pressed-wood-products-safety-factsheet - Binetti, Roberto, Francesca Marina Costamagna, and Ida Marcello. "Development of carcinogenicity classifications and evaluations: the case of formaldehyde." ANNALI-ISTITUTO SUPERIORE DI SANITA 42, no. 2 (2006): 132.
- Brelih, Nejc, and Olli Seppänen. "Ventilation rates and IAQ in European standards and national regulations." In The proceedings of the 32nd AIVC conference and 1st TightVent conference in Brussels, pp. 12-13. 2011.
- Bruynzeel, Derk P., Klaus E. Andersen, José G. Camarasa, Jean‐Marie Lachapelle, Torkil Menné, and Ian R. White. "The European standard series." Contact Dermatitis 33, no. 3 (1995): 145-148.
- Brown, S. K. "Chamber Assessment of Formaldehyde and VOC Emissions from Wood‐Based Panels." Indoor air 9, no. 3 (1999): 209-215.
- European Chemicals Agency (ECHA), "Response to comments document (RCOM)
to the Opinion proposing harmonised classification and
labelling at EU level of
Formaldehyde", Committee for Risk Assessment (RAC) of the ECHA, (2012), retrieved 29 March 2015, original source: http://echa.europa.eu/documents/10162/e38c0fba-6104-4646-b787-116ed63deb9d - Harald Schwab, Rainer Marutzky, Bettina Meyer, "European Regulations for Formaldehyde", [PDF] Fraunhofer Institute for Wood Research
Wilhelm-Klauditz-Institut
Braunschweig / Germany, Retrieved 29 March 2015, original source http://owic.oregonstate.edu/sites/default/files/pubs/Schwab.pdf - Groah, William J., John Bradfield, Gary Gramp, Rob Rudzinski, and Gary Heroux. "Comparative response of reconstituted wood products to European and North American test methods for determining formaldehyde emissions." Environmental Science & Technology 25, no. 1 (1991): 117-122.
- Kim, Sumin, Jin-A. Kim, Hyun-Joong Kim, and Shin Do Kim. "Determination of formaldehyde and TVOC emission factor from wood-based composites by small chamber method." Polymer Testing 25, no. 5 (2006): 605-614.
- Kim, Sumin, and Hyun-Joong Kim. "Comparison of standard methods and gas chromatography method in determination of formaldehyde emission from MDF bonded with formaldehyde-based resins." Bioresource Technology 96, no. 13 (2005): 1457-1464.
- Lund, Kirsten H., and Jens Højslev Petersen. "Migration of formaldehyde and melamine monomers from kitchen-and tableware made of melamine plastic." Food additives and contaminants 23, no. 9 (2006): 948-955.
- Salthammer, Tunga, Sibel Mentese, and Rainer Marutzky. "Formaldehyde in the indoor environment." Chemical Reviews 110, no. 4 (2010): 2536-2572.
- Tang, Xiaojiang, Yang Bai, Anh Duong, Martyn T. Smith, Laiyu Li, and Luoping Zhang. "Formaldehyde in China: Production, consumption, exposure levels, and health effects." Environment international 35, no. 8 (2009): 1210-1224.
Canadian Formaldehyde Exposure Standards
"The risk of developing cancer from formaldehyde levels found in Canadian homes ... is essentially zero" - Health Canada
- Health Canada, "Formaldehyde in Indoor Air", Health Canada . Sante Canada, retrieved 29 March 2015, original source: http://www.hc-sc.gc.ca/ewh-semt/pubs/air/formaldehyde/fact-info-eng.php
Excerpt:
Although formaldehyde is a known carcinogen, the risk of developing cancer from formaldehyde exposure at concentrations found in most Canadian homes is essentially zero.
Formaldehyde can be found in the air of most, if not all, homes.
It is, however, generally below the levels recommended by Health Canada. Formaldehyde may be a concern for people with respiratory problems (such as children with asthma) following new home construction or renovations, when levels are generally at their highest.
Health Canada's Residential Indoor Air Quality Guideline for formaldehyde recommends maximum exposure limits of;- Short-term Formaldehyde exposure: 123 µg/m³ (100 ppb) based on a 1-hour average to protect against irritation of the eyes, nose or throat.
- Long-term Formaldehyde exposure: 50 µg/m³ (or 40 ppb) based on a minimum 8-hour average, to protect against respiratory symptoms in children with asthma.
Health Canada's recommended short-term exposure limit is 1/10th of the lowest level at which symptoms have been observed, in order to protect more sensitive individuals. In direct exposure studies, formaldehyde has been shown to cause irritation of the eyes, nose and throat at 1230 µg/m³ (1000 ppb). However, people may vary in their sensitivity to formaldehyde and some may experience symptoms at lower levels.
Health Canada's recommended long-term exposure limit is set to protect children with asthma, who may be more sensitive to the effects of formaldehyde. Long-term exposure to formaldehyde in indoor air has been associated with increased allergic sensitivity, airway inflammation and physician-diagnosed asthma.
Health Canada's recommended levels also protect against the potential cancer risk from formaldehyde. Formaldehyde is classified as "carcinogenic to humans" by the International Agency for Research on Cancer (IARC). Industrial workers exposed to high levels of formaldehyde as part of their jobs have shown a higher risk of developing rare forms of cancer that affect the upper respiratory systems. The risk of developing cancer from formaldehyde levels found in Canadian homes, however, is essentially zero.
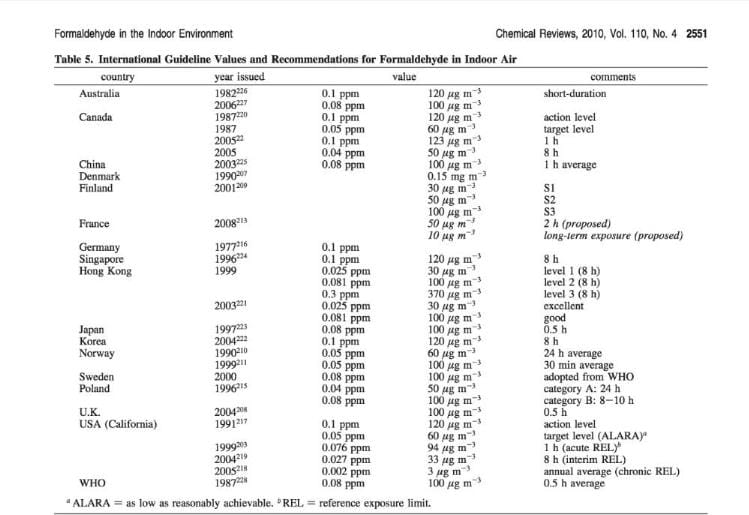
Table of Formaldehyde Exposure Recommendations, World Wide
The following table is excerpted from Salthammer, Tunga, Sibel Mentese, and Rainer Marutzky. "Formaldehyde in the indoor environment." Chemical Reviews 110, no. 4 (2010): 2536-2572. More work by these authors is cited atReferences or Citations .
[Click to enlarge any image]
In comparing the recommended exposure limits for formaldehyde in various countries around the world as given in the table below note that the conversions from ppm are given in micrograms: ug per m-3 NOT milligrams: mg/m3 (micrograms per cc rather than milligrams per cubic meter).
Calculate Conversions Between PPM and mg/m3
How & When to Convert Exposure Limits in ppm to mg/m3 for Formaldehyde or other Substances
Concentrations of chemicals in air are typically measured in units of the mass of chemical (milligrams, micrograms, nanograms, or picograms) per volume of air (cubic meter or cubic feet). However, concentrations may also be expressed as parts per million (ppm) or parts per billion (ppb) by using a conversion factor. The conversion factor is based on the molecular weight of the chemical and is different for each chemical. Also, atmospheric temperature and pressure affect the calculation. - Boguski (2006)
Only concentrations of substances that exist as a gas or vapor in air at "normal" room temperatures and pressures can be expressed as parts per million. In order to be able to express exposure limits in a consistent manner across a variety of materials, including metals, salts or other substances that are not airborne as vapors, occupational exposure limits are typically given as milligrams per cubic meter of air or mg/m3 of a substance in air. Still other substances such as airborne asbestos or fiberglass fibers may be expressed as fibers / cc but we warn that "fibers" needs to be more carefully defined since the size of particles of these materials can vary significantly and as health implications may also vary by particle size.
The Canadian Centre for Occupational Health and Safety (CCOHS) (www.ccohs.ca) and the ACGIH ("Threshold Limit Values TLVs™) discuss both threshold limit values (TLVs™) and Biological Exposure Indices (BEIs™) and give formulas for TLVs in both ppm and in mg/m3 assuming that these measurements are made at 25C and 1 atmosphere of pressure (1 ATM = 760 torr = 760 mm Hg = 406.64355 inches of water).
One Gram Molecular Weight or GMW or "mole" can be defined as the quantity of a substance whose weight in grams is equal to the molecular weight of that substance
Moles or molecular weights of any substance are calculated as
Atomic Weight of a Substance (from the periodic chart of its comprising elements expressed in atomic mass units) x number of atoms of each element present = the formula weight of the substance.
So any GMW or mole calculations for any substance require that you know the atomic weight of its individual comprising atoms as well as the number of atoms making up substance. The atomic weights of elements are found in a periodic table or in tables maintained by NIST.
Formulas for Converting Concentration of a Substance Between ppm and mg/M3
Assuming a pressure of 1 ATM and a temperature of 25C, we can write equations to convert between concentrations of a substance in parts per million (ppm) to concentration of the same substance in mg/M3 as follows: - Boguski (2006)
To convert ppm to mg/m3
Concentration of X (mg/m3) = 0.0409 x concentration of X (ppm) x molecular weight of X
To convert mg/m3 to ppm
Concentration (ppm) = 24.45 x concentration (mg/m3) ÷ molecular weight of X
Formaldehyde's atomic weight is 30.02598
To avoid confusion between milligrams per cubic meter and micrograms per cubic meter: 1.0 mg/m3 = 1000.0 ug/m3
Examples of Formaldehyde Concentration Conversions between ppm and mg/M3 at 1 ATM and 25°C |
||
| Formaldehyde Level in ppm | Formaldehyde Level in mg/m3 | Notes |
| 3 ppm | 3.684 mg/M3 | |
| 1 ppm | 1.23 mg/M3 | |
| 0.5 ppm | 0.6 mg/M3 | |
| 0.1 ppm | 0.123 mg/M3 | Checkpoint with table 5 from Salthammer quoting Chemical Reviews and given above |
| 0.01 ppm | 0.0123 mg/M3 | |
| 325.72 ppm | 400 mg/M3 | ppm calculated per Example 2 in Notes below, or 303 ppm using Lenntech's calculator |
| 165.3 ppm | 203 mg/M3 | |
Notes to the Table Above
Use these formulas to convert between ppm and mg/m3 assuming that the measurement is made at 1 ATM and 25°C (These numbers will be incorrect at other temperatures or pressures. )
0.0409 x concentration of Formaldehyde (in ppm) x molecular weight of Formaldehyde = Concentration of Formaldehyde in (mg/m3)
Example#1: convert 3 ppm of formaldehyde to mg/m3
0.0409 x 3ppm HCHO x 30.02598-HCHO = 3.68418775 mg/m3
Using the molecular weight of formaldehyde = 30.02598
Example #2: convert 400 mg/m3 of formaldehyde to ppm
24.45 x concentration 400 (mg/m3) ÷ 30.02598 molecular weight of Formaldehyde = 325.7179283 Concentration (ppm)
If you are working in micrograms per cubic meter (ug/m3) instead of milligrams per cubic meter (mg/m3) you can use these same equations to obtain parts per billion (ppb) instead of parts per million (ppm).
If you are working in concentrations of chemical substances in water the usual measurements are in mg/L or ug/L.
Just to keep our decimal places straight, if you are converting between ppm and ppb, 1 ppm = 1,000 ppb. A milligram is 1/1000 of a gram or 0.001 grams; a nanogram is 1/1,000,000,000 grams or a billionth of a gram, or 0.000000001 grams.
Definition & Composition of the molecular mass (popularly "weight") of Formaldehyde
The molecular mass of any substance is the sum of the mass of its constituent atoms. To have a useful meaning, all atomic mass units are expressed relative to the isotope 12C which is defined to have a mass of 12. Formaldehyde has an atomic weight expressed in atomic mass units or molar mass of 30.02598 computed as follows:
What is the atomic mass of Formaldehyde?
A molecule of formaldehyde is made up of a combination of atomic elements written as CH2O (or HCHO)
Atomic mass of H, Hydrogen = 1.00794
Atomic mass of C, Carbon = 12.0107
Atomic mass of O, Oxygen = 15.9994
These atomic mass units or popularly, "atomic weights"are given in the atomic table. Adding these for CH2O = (H + H + C + O) = (1.00794+1.00794+12.0107+15.9994) = 30.02598
What is one gram molecular weight of Formaldehyde? What is one mole of Formaldehyde?
One gram Molecular Weight (GMW) of any substance is defined as the amount of the substance whose weight in grams is numerically identical to the molecular weight of that substance.
One gram molecular weight (1 GMW) of formaldehyde = 30.02598 grams.
How many grams of formaldehyde are in one mole of that substance? The same. The term "mole" is a synonym for "gram molecular weight". One mole of CH2O = 30.02598 grams.
Or inversely, one gram of formaldehyde = 0.0333044916436 moles.
How Gram Molecular Weight is used in TLV (Threshold Limit Value) calculations
Threshold limit values (TLVs), if we know the GMW of a substance, can let us easily convert between ppm and mg/m3 with the warning that the measurements assume that environment where the measurement is made is at 1ATM of pressure (sea level) and at 25°C. At other pressures and temperatures more adjustments need to be made to the simplified calculations shown below.
Any TLV expressed in mg/m3 = (gram molecular weight of substance) x (TLV in ppm) / 24.45
Any TLV expressed in ppm = 24.45 x (TLV in mg/m3) / (gram molecular weight of substance)
where 24.5 is a constant that equals the volume in liters of one mole (or one GMW) of a gas or vapor at 1 ATM of pressure at a temperature of 25C. For other temperatures the molecular weight must be re-calculated using the gas laws: V=(RT/P) where V=volume, R is the ideal gas constant, T is temperature in Kelvins, P is pressure in mm of hg.
Background about Gram Molecular Weight, Moles, Avogrado's Constant, and Fork Union Military Academy's Captain Peterson
In high school chemistry at Fork Union Military Academy Captain Peterson (ca 1959-1960) taught us that
Avogrado's number, 6.0221413 x 1023
is defined as the mass of one mole (one gram molecular weight) of any substance. He also used as an equivalent term "gram molecule" or "gram molecular weight" for "mole" but Peterson is long retired and gram-molecule has lost popularity to a somewhat different term "gram atom".
One gram-atom or "gat.", a somewhat different idea, is the quantity of any substance that contains Avogrado's number of atoms.
Peterson, when he, along with the science teacher, was not busy building a still in the top floor chemistry lab, was an exciting teacher who was inclined put a little more lycopodium powder into a can with a candle (bigger boom) or to cut a little larger piece of raw sodium metal to toss into the Fork Union sewer drain (which was tremendously exciting).
When a spider was slowly spinning its way down over his desk, Peterson calmly opened a cylinder of raw chlorine gas that he had handy - just a little squirt, that's all. The spider turned white and the students, choking on chlorine, all jumped out of the classroom windows.
Online calculator converts among μg/L, μg/m3, ppmV and % concentrations
The U.S. EPA provides "EPA On-Line Tools for Site Assessment Calculation" at http://www.epa.gov/athens/learn2model/part-two/onsite/ia_unit_conversion.html including a calculator that converts measurements among various scales such as μg/L, μg/m3, ppmV and %.
However regrettably formaldehyde is not among the gases included in the EPA's calculator. We have asked that the EPA add that substance.
- "Boguski, Terrie K., "Understanding Units of Measurement", Center for Hazardous Substance Research, Kansas State University, (October 2006), Terrie K. Boguski, P.E., is the Assistant Technical Director of the CHSR at Kansas State University (tboguski@ksu.edu). - retrieved 18 April 2015, original source https://www.ksutab.org/?ResponseView=TABResourceDownloadView&id=9
Formaldehyde Outgassing from Clothing & Soft goods: Exposure Limits
According to the New York Times, the most stringent (but voluntary) industry standards for exposure to formaldehyde were established in Japan, as follows:
- 20 ppm or less (down to "undetectable") for products that touch the skin of children under three years of age
- 75 ppm for products that touch the skin of people other than children under 3 year old
- 300 ppm for products that do not touch the skin (such as outerwear)
- FORMALDEHYDE in TEXTILES, U.S. G.A.O. report to Congress. While [formaldehyde] levels in clothing generally appear to be low, allergic contact dermatitis is a health issue for some people, GAO-10-875, August 2010, retrieved 12/13/2010, original source: http://www.gao.gov/new.items/d10875.pdf
OPINION - DF: We liked this report as exemplary in thoroughness, clarity, and impartiality on the subject of formaldehyde exposure from textiles.
According to the NY Times article cited above, some critics complained that the U.S. government study should have included a wider arrange of textiles such as draperies and upholstery, and we agree, but add that upholstered furniture may in some products outgas more formaldehyde from foam used in the product than from its covering fabric. Similarly, carpeting could have been considered.
But the focus of the study was textiles that are likely to be in prolonged (rather than incidental) contact with human skin. Under the aegis of contact with human skin and recognizing the very low limit set in Japan for fabrics in contact with the skin of children under three, one can but wonder about the possibility of concerns for skin contact of infants and small children placed on carpeting.
References:
- European Commission Joint Research Centre, "European Survey on the Release of FOrmaldehyde from Textiles", EC JRC (2007), European Commission
Directorate-General Joint Research Centre
Institute for Health and Consumer Protection
Contact information
Address: via E. Fermi 1, 21020 Ispra (VA), Italy
E-mail: paola.piccinini@jrc.it
Tel.: 0039-0332-789124
EUR 22710 EN
ISBN 978-92-79-05215-6
ISSN 1018-5593
Luxembourg: Office for Official Publications of the European Communities,
Website: http://www.jrc.cec.eu.int/pce http://www.jrc.cec.eu.int , retrieved 29 march 2015, original source: http://publications.jrc.ec.europa.eu/repository/bitstream
/111111111/5233/1/6150%20-%20HCHO_survey_final_report.pdf
InspectAPedia.com is an independent publisher of building, environmental, and forensic inspection, diagnosis, and repair information for the public - we have no business nor financial connection with any manufacturer or service provider discussed at our website.
...
Reader Comments, Questions & Answers About The Article Above
Below you will find questions and answers previously posted on this page at its page bottom reader comment box.
Reader Q&A - also see RECOMMENDED ARTICLES & FAQs
Question: report on inadequate indoor gas testing, deaths, illnesse, worries about competent gas testing
My wife and I were exposed to carbon monoxide (and I'm assuming other combustible gases) for well over a year without knowing. We lived on a ground floor of an apartment and holes were drilled through the floor for plumbing and elec. but were never covered. They insurance company had Pinchin Engineering do a test on the place. and we were shown a basic 1 page sheet of CO and CO2 levels both in and around the house.
The readings from our detector that we finally bought a year later were at times almost 300. there readings were 20.
however the CO2 levels were 859.
Does this mean that combustion was happening because of both the readings and if so wouldn't tests be done for other combustible gases that may be present?
We lost a baby, dog died 2 years later, heart and kidney failure. wife has heart and joint issues. and I have a list as well. They settled out of court for $300Gs but I feel like we didn't receive ALL the disclosure from the air quality test. - Matt 11/30/11
Reply:
Matt,
Of course we are so sorry about the tragic losses you described, and I understand the tragedy of losing a child. When we suffer a tragic loss it is so difficult to endure that the look for a cause and blame is understandable, though sometimes we can be led astray in such a search.
In attempting to relate an illness or fatality to a building or environmental condition, a good place to start is with the physicians involved - ask about possible relationships between the illness or death and environmental factors.
About varying gas levels in buildings, our field experience confirms that small changes in a building (a window open or shut, or a door, or a bird building a nest in a chimney, or seasonal sooting in an oil fired appliance) and similar events can make an enormous difference in the measured result of indoor gases, airborne mold, other contaminants. For that reason, it is no surprise that a year later an individual measurement of gases indoors might find a very different level.
Only if the source of an indoor hazard can be clearly traced to a condition that was present, recogniziable by general home inspection standards and procedures, and visible at the time of the original inspection would one suspect the original inspector or test company of negligence.
- For CO2 (carbon dioxide) typical indoor levels see TYPICAL CO2 LEVELS.
- For CO (carbon monoxide) information see CARBON MONOXIDE - CO
- For examples of the sorts of conditions that an inspector should be able to observe and report
see CARBON MONOXIDE INSPECTION
- Lastly, when considering a report from any building investigator, make certain that s/he is financially and ethicallyi completely independent from the person or company recommending or arranging for that service or inspection. Conflicts of interest in such matters are intolerable and even dangerous. And of course, provding more than one version of any building investigation report, say different versions to different parties, smells bad to me too.
...
Continue reading at FORMALDEHYDE GAS TEST KITS, METERS, or select a topic from the closely-related articles below, or see the complete ARTICLE INDEX.
Or see these
Recommended Articles
- FORMALDEHYDE HAZARDS - home
- FORMALDEHYDE GAS EXPOSURE LIMITS
- FORMALDEHYDE GAS HAZARD REDUCTION
- FORMALDEHYDE GAS SOURCES in BUILDINGS
- FORMALDEHYDE GAS SOURCES & HAZARDS RESEARCH
- FORMALDEHYDE GAS TEST KITS, METERS
- FORMALDEHYDE GAS TEST METHODS, PROCEDURES
- FORMALDEHYDE in LAMINATE FLOORING
- UREA FORMALDEHYDE FOAM INSULATION, UFFI
Suggested citation for this web page
FORMALDEHYDE GAS EXPOSURE LIMITS at InspectApedia.com - online encyclopedia of building & environmental inspection, testing, diagnosis, repair, & problem prevention advice.
Or see this
INDEX to RELATED ARTICLES: ARTICLE INDEX to GAS HAZARDS in BUILDINGS
Or use the SEARCH BOX found below to Ask a Question or Search InspectApedia
Ask a Question or Search InspectApedia
Try the search box just below, or if you prefer, post a question or comment in the Comments box below and we will respond promptly.
Search the InspectApedia website
Note: appearance of your Comment below may be delayed: if your comment contains an image, photograph, web link, or text that looks to the software as if it might be a web link, your posting will appear after it has been approved by a moderator. Apologies for the delay.
Only one image can be added per comment but you can post as many comments, and therefore images, as you like.
You will not receive a notification when a response to your question has been posted.
Please bookmark this page to make it easy for you to check back for our response.
IF above you see "Comment Form is loading comments..." then COMMENT BOX - countable.ca / bawkbox.com IS NOT WORKING.
In any case you are welcome to send an email directly to us at InspectApedia.com at editor@inspectApedia.com
We'll reply to you directly. Please help us help you by noting, in your email, the URL of the InspectApedia page where you wanted to comment.
Citations & References
In addition to any citations in the article above, a full list is available on request.
- AIHA, American Industrial Hygiene Association, "Is Formaldehyde from Laminate Flooring a Problem in My Home?", AIHA [draft] 8 May 2015, copy on file.
- "Boguski, Terrie K., "Understanding Units of Measurement", Center for Hazardous Substance Research, Kansas State University, (October 2006), Terrie K. Boguski, P.E., is the Assistant Technical Director of the CHSR at Kansas State University (tboguski@ksu.edu). - retrieved 18 April 2015, original source https://www.ksutab.org/?ResponseView=TABResourceDownloadView&id=9
- U.S. Environmental Protection Agency. Health and Environmental Effects Profile for Formaldehyde. EPA/600/x-85/362. Environmental Criteria and Assessment Office, Office of Health and Environmental Assessment, Office of Research and Development, Cincinnati, OH. 1988.
- American Home Furnishing Alliance (AHFA), 1912 Eastchester Drive, Suite 100 High Point, North Carolina 27265, Website: https://www.ahfa.us/ Excerpt: The American Home Furnishings Alliance is the voice of the residential furniture industry, representing companies large and small, public and private, domestic and import.
- U.S. Environmental Protection Agency. Health and Environmental Effects Profile for Formaldehyde. EPA/600/x-85/362. Environmental Criteria and Assessment Office, Office of Health and Environmental Assessment, Office of Research and Development, Cincinnati, OH. 1988.
- World Health Organization. Environmental Health Criteria for Formaldehyde. Volume 89. World Health Organization, Geneva, Switzerland. 1989.
- U.S. Department of Health and Human Services. Registry of Toxic Effects of Chemical Substances (RTECS, online database). National Toxicology Information Program, National Library of Medicine, Bethesda, MD. 1993.
- E.J. Calabrese and E.M. Kenyon. Air Toxics and Risk Assessment. Lewis Publishers, Chelsea, MI. 1991.
- U.S. Department of Health and Human Services. Hazardous Substances Databank (HSDB, online database). National Toxicology Information Program, National Library of Medicine, Bethesda, MD. 1993.
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Formaldehyde (Draft). Public Health Service, U.S. Department of Health and Human Services, Atlanta, GA. 1997.
- J.E. Amoore and E. Hautala. Odor as an aid to chemical safety: Odor thresholds compared with threshold limit values and volatilities for 214 industrial chemicals in air and water dilution. Journal of Applied Toxicology, 3(6):272-290. 1983.
- American Conference of Governmental Industrial Hygienists (ACGIH). 1999 TLVs and BEIs. Threshold Limit Values for Chemical Substances and Physical Agents, Biological Exposure Indices. Cincinnati, OH. 1999.
- National Institute for Occupational Safety and Health (NIOSH). Pocket Guide to Chemical Hazards. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention. Cincinnati, OH. 1997.
- Occupational Safety and Health Administration (OSHA). Occupational Safety and Health Standards, Toxic and Hazardous Substances. Code of Federal Regulations 29 CFR 1910.1048. 1998.
- American Industrial Hygiene Association (AIHA). The AIHA 1998 Emergency Response Planning Guidelines and Workplace Environmental Exposure Level Guides Handbook. 1998.
- Riess, Ulrich, Uwe Tegtbur, Christian Fauck, Frank Fuhrmann, Doreen Markewitz, and Tunga Salthammer. "Experimental setup and analytical methods for the non-invasive determination of volatile organic compounds, formaldehyde and NO x in exhaled human breath." Analytica chimica acta 669, no. 1 (2010): 53-62.
- Salthammer, Tunga. "Formaldehyde in the ambient atmosphere: from an indoor pollutant to an outdoor pollutant?." Angewandte Chemie International Edition 52, no. 12 (2013): 3320-3327
- Salthammer, Tunga, Sibel Mentese, and Rainer Marutzky. "Formaldehyde in the indoor environment." Chemical Reviews 110, no. 4 (2010): 2536-2572.
- Salthammer, T., F. Fuhrmann, S. Kaufhold, B. Meyer, and A. Schwarz. "Effects of climatic parameters on formaldehyde concentrations in indoor air." Indoor Air 5, no. 2 (1995): 120-128.
- In addition to citations & references found in this article, see the research citations given at the end of the related articles found at our suggested
CONTINUE READING or RECOMMENDED ARTICLES.
- Carson, Dunlop & Associates Ltd., 120 Carlton Street Suite 407, Toronto ON M5A 4K2. Tel: (416) 964-9415 1-800-268-7070 Email: info@carsondunlop.com. Alan Carson is a past president of ASHI, the American Society of Home Inspectors.
Thanks to Alan Carson and Bob Dunlop, for permission for InspectAPedia to use text excerpts from The HOME REFERENCE BOOK - the Encyclopedia of Homes and to use illustrations from The ILLUSTRATED HOME .
Carson Dunlop Associates provides extensive home inspection education and report writing material. In gratitude we provide links to tsome Carson Dunlop Associates products and services.