 Well Disinfectant Choices
Well Disinfectant Choices
- POST a QUESTION or COMMENT about properties of alternative well disinfectants.
Well disinfectant chemicals & their properties:
This article describes the properties of three most common disinfectants used to shock or sanitize a water well: chlorine (common household bleach), chloramine, and chlorine dioxide.
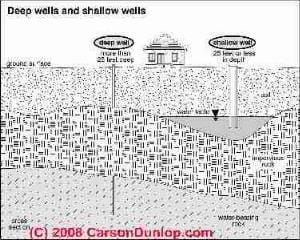
Page top sketch illustrating both deep and shallow water well construction and depths is provided courtesy of Carson Dunlop Associates, a Toronto home inspection, education & report writing tool company [ carsondunlop.com ].
InspectAPedia tolerates no conflicts of interest. We have no relationship with advertisers, products, or services discussed at this website.
- Daniel Friedman, Publisher/Editor/Author - See WHO ARE WE?
Comparison of the 3 Common Well Water Disinfectants: Chloramine, Chlorine and Chlorine Dioxide
Chloramine, Chlorine and Chlorine Dioxide Used for Well Shocking / Disinfection Procedures |
|||
| Disinfectant | Common Usages | Health Effects | Comments |
| Chloramine (as Cl2) | Chloramine is a water additive used to control microbes ... as a residual disinfectant in drinking water distribution system pipes. Chloramine is formed when ammonia is added to water containing free chlorine. Monochloramine is a form of chloramine commonly used for disinfection by municipal water systems |
Drinking water with excessive levels of chloramine above the maximum residual disinfectant level (MRDL) could experience irritating effects to their eyes and nose, stomach discomfort or anemia | MRDL = 4.0 mg/L or 4 ppm as an annual average |
| Chlorine (as Cl2) | As a gaseous or liquid form of chlorine (CL2) chlorine is a powerful oxidant used by municipal water systems to control microbes. Chlorine is relatively inexpensive and has the lowest production and operating costs and longest history for large continuous disinfection operations. |
Some people who use water containing chlorine well in excess of the maximum residual disinfectant level could experience irritating effects to their eyes and nose. Some people who drink water containing chlorine well in excess of the maximum residual disinfectant level could experience stomach discomfort. |
MRDL = 4.0 mg/L or 4 ppm as an annual average |
| Chlorine dioxide (as ClO2) | Chlorine dioxide is added to water to control microbes and can be used to control tastes and odors. Highly volatile, chlorine dioxide rapidly disappears from stored water. | Some infants, young children, and fetuses of pregnant women who drink water containing chlorine dioxide in excess of the maximum residual disinfectant level could experience nervous system effects . Some people who drink water containing chlorine dioxide well in excess of the MRDL for many years may experience anemia. |
MRDL = 0.8 mg/L or 800 ppb |
| Household Bleach e.g. Clorox® contents & purposes of each chemical |
|
According to the US ATSDR's fact sheet on chlorine: Watch out: Drinking small amounts of hypochlorite solution (less than a cup) can produce irritation of the esophagus. Drinking concentrated hypochlorite solution can produce severe damage to the upper digestive tract and even death. These effects are most likely caused by the caustic nature of the hypochlorite solution and not from exposure to molecular chlorine. Spilling hypochlorite solution on the skin can produce irritation. The severity of the effects depends on the concentration of sodium hypochlorite in the bleach. |
Adapted from clorox.com For emergency disinfection of drinking water (his is NOT the same thing as disinfecting a well) add 8 drops of Clorox bleach to a gallon of clear water, (or just two drops to a quart) and let the solution stand for at least 15 minutes (a very slight "bleach" odor should remain. If there is no bleach odor the water may not be adequately disinfected. See DRINKING WATER EMERGENCY PURIFICATION
|
Notes to the table above
The above information is adapted from US EPA, Water: Basic Information about Regulated Drinking Water Contaminants, retrieved 8/27/2013, original source water.epa.gov/drink/contaminants/basicinformation/disinfectants.cfm [1]
and from information provided by clorox.com
Watch out: The US EPA recommends that you test your water every year for total
coliform bacteria, nitrates, total
dissolved solids, and pH levels.
If a review of local well water contaminants found by local testing labs, your local health department, or other information about your particular property suggests that there is a risk of other chemical contaminants (such as agricultural chemicals, pesticides, or local chemical spills or dumps) then you should include a test for these contaminants as well.
For problem wells whose contamination is not easily cured by the above, see WELL DISINFECTANT TABLE, POST FLOODING
...
Continue reading at WELL CHLORINATION SHOCKING PROCEDURE or select a topic from the closely-related articles below, or see the complete ARTICLE INDEX.
Or see these
Recommended Articles
- CHEATING ON WATER TESTS
- DUG WELL SANITATION PROTECTION
- FAILED WATER TESTS - WHEN to RE-TEST
- FLOOD CONTAMINATED WELL RESTORE & PROTECT
- WATER TEST INTERPRETATION
- WATER TREATMENT EQUIPMENT CHOICES - home
- WATER WELL CONTAMINATION HAZARDS
- WATER WELL SAFETY & SANITATION, 6 STEPS-EPA
- WELL CHLORINATION & DISINFECTION
- WELL CHLORINATION DRIVEN SAND POINT
- WELL CLEANING PROCEDURES
- WELL CLEANING BY GLYCOLIC ACID - remove biofilms, scale, restore well flow
- WELL DYNAMIC HEAD & STATIC HEAD DEFINITION - table of well water volumes
- WELL DISINFECTANT CHOICES
- WELL DISINFECTANT pH ADJUSTMENT - to be sure your well treatment is effective
- WELL DISINFECTION PROCEDURE, POST FLOODING
Suggested citation for this web page
WELL DISINFECTANT CHOICES at InspectApedia.com - online encyclopedia of building & environmental inspection, testing, diagnosis, repair, & problem prevention advice.
Or see this
INDEX to RELATED ARTICLES: ARTICLE INDEX to WATER TREATMENT SYSTEMS
Or use the SEARCH BOX found below to Ask a Question or Search InspectApedia
Ask a Question or Search InspectApedia
Try the search box just below, or if you prefer, post a question or comment in the Comments box below and we will respond promptly.
Search the InspectApedia website
Note: appearance of your Comment below may be delayed: if your comment contains an image, photograph, web link, or text that looks to the software as if it might be a web link, your posting will appear after it has been approved by a moderator. Apologies for the delay.
Only one image can be added per comment but you can post as many comments, and therefore images, as you like.
You will not receive a notification when a response to your question has been posted.
Please bookmark this page to make it easy for you to check back for our response.
Our Comment Box is provided by Countable Web Productions countable.ca
Citations & References
In addition to any citations in the article above, a full list is available on request.
- [1]US EPA, "Water: Basic Information about Regulated Drinking Water Contaminants", retrieved 8/27/2013, original source http://water.epa.gov/drink/contaminants/basicinformation/disinfectants.cfm
- [1a] Drinking Water from Household Wells - PDF, U.S. EPA, Original source last retrieved 2/13/2013, original source: http://www.epa.gov/privatewells/pdfs/household_wells.pdf
- [1b] US EPA, "Chlorine Dioxide" (Alternative Water Disinfectants Manual) [PDF] U.S. Environmental Protection Agency
- [2] ATSDR Agency for Toxic Substances Disease Registry, Toxic Substances Portal: Chlorine Toxicity, [PDF] November 2010, retrieved 8/27/2013, original source: www.atsdr.cdc.gov
- [2a]Thanks to reader Jerry Highsmith for discussing well shocking procedures where a water filter or water softener are installed - August 2010
- [3] "Bacteria in Drinking Water" - "Chlorine," Karen Mancl, water quality specialist, Agricultural Engineering, Ohio State University Extension. Mancl explains factors affecting the effectiveness of chlorine in water as a means to destroy bacteria and other microorganisms. OSU reports as follows:
Chlorine kills bacteria, including disease-causing organisms and the nuisance organism, iron bacteria. However, low levels of chlorine, normally used to disinfect water, are not an effective treatment for giardia cysts. A chlorine level of over 10 mg/1 must be maintained for at least 30 minutes to kill giardia cysts. -- http://ohioline.osu.edu/b795/index.html is the front page of this bulletin.
- [5] Canadian BC Ministry of Environment, "Water Stewardship Information Series: When Standard Water Well Chlorination Procedures are Ineffective Fact Sheet developed for Well Drillers, Health Authority Staff and others involved in well recovery efforts after a flood " ", Agriculture and Agri-Food, Canada, retrieved 4/8/2013 original source: http://www.env.gov.bc.ca/wsd/plan_protect_sustain/ groundwater/wells/factsheets/PFRA_well_recovery.pdf
- [5a] "Chemical Cleaning, Disinfection and Decontamination of Water Well" John Schnieders. Johnson Screens Inc. St. Paul, MN
- [5b] Water Well Management Level 2 Training Module. Prairie Farm Rehabilitation Administration of Agriculture and Agri-Food Canada, Alberta Environment, Alberta Water Well Drilling Association and Alberta Agriculture, Food and Rural Development
- [6] Water Wells that last for Generations. Alberta Agriculture, Food and Rural Development; Alberta Environment; Prairie Farm Rehabilitation Administration of Agriculture and Agri-Food Canada
- [7] Water Well Management Level 2 Training Module. Prairie Farm Rehabilitation Administration of Agriculture and Agri-Food Canada, Alberta Environment, Alberta Water Well Drilling Association and Alberta Agriculture, Food and Rural Development
- [8] Chemical Cleaning, Disinfection and Decontamination of Water Wells. John Schnieders. Published by Johnson Screens Inc. St. Paul, MN
- Ohio State University article on the concentration of chlorine necessary to act as an effective disinfectant, and the effects of the water's pH and temperature: See http://ohioline.osu.edu/b795/b795_7.html for details.
- Water Quality Association P.O. Box 606 4151 Naperville Road Lisle, IL 60532 www.wqa.org
- National Sanitation Foundation P.O. Box 130140 789 N Dixboro Road Ann Arbor, MI 48113-0140 (734) 769-8010, (800) NSF-MARK www.nsf.org
- U.S. Environmental Protection Agency (to visit in person) Office of Water Resource Center 1200 Pennsylvania Avenue, NW Ariel Rios Building Washington, DC 20460 Phone: (202) 260-7786, email: center.water-resource@epa.gov
- The Safe Drinking Water Hotline (800) 426-4791 The hotline operates from 9:00 AM to 5:30 PM (EST) The hotline can be accessed on the Internet at www.epa.gov/safewater/drinklink.html
- Water Systems Council www.watersystemscouncil.org
- Our recommended books about building & mechanical systems design, inspection, problem diagnosis, and repair, and about indoor environment and IAQ testing, diagnosis, and cleanup are at the InspectAPedia Bookstore. Also see our Book Reviews - InspectAPedia.
- Crystal Clear Supply provides portable ceramic water filter purifiers and portable reverse osmosis water treatment equipment - see http://www.crystalclearsupply.com/category_s/7.htm
- Handbook of Disinfectants and Antiseptics, Joseph M. Ascenzi (Editor), CRC, 1995, ISBN-10: 0824795245 ISBN-13: 978-0824795245 "The evaluation of chemical germicides predates the golden age of microbiology..." -
This well-focused, up-to-date reference details the current medical uses of antiseptics and disinfectants -- particularly in the control of hospital-acquired infections -- presenting methods for evaluating products to obtain regulatory approval and examining chemical, physical, and microbiological properties as well as the toxicology of the most widely used commercial chemicals. - Potable Aqua® emergency drinking water germicidal tablets are produced by the Wisconsin Pharmacal Co., Jackson WI 53037. 800-558-6614 pharmacalway.com
- Principles and Practice of Disinfection, Preservation and Sterilization (Hardcover)
by A. D. Russell (Editor), W. B. Hugo (Editor), G. A. J. Ayliffe (Editor), Blackwell Science, 2004. ISBN-10: 1405101997, ISBN-13: 978-1405101998.
"This superb book is the best of its kind available and one that will undoubtedly be useful, if not essential, to workers in a variety of industries. Thirty-one distinguished specialists deal comprehensively with the subject matter indicated by the title ... The book is produced with care, is very readable with useful selected references at the end of each chapter and an excellent index. It is an essential source book for everyone interested in this field. For pharmacy undergraduates, it will complement the excellent text on pharmaceutical microbiology by two of the present editors."
The Pharmaceutical Journal: "This is an excellent book. It deals comprehensively and authoritatively with its subject with contributions from 31 distinguished specialists. There is a great deal to interest all those involved in hospital infection ... This book is exceptionally well laid out. There are well chosen references for each chapter and an excellent index. It is highly recommended." The Journal of Hospital Infection.: "The editors and authors must be congratulated for this excellent treatise on nonantibiotic antimicrobial measures in hospitals and industry ... The publication is highly recommended to hospital and research personnel, especially to clinical microbiologists, infection-control and environmental-safety specialists, pharmacists, and dieticians."
New England Journal of Medicine: City Hospital, Birmingham, UK. Covers the many methods of the elimination or prevention of microbial growth. Provides an historical overview, descriptions of the types of antimicrobial agents, factors affecting efficacy, evaluation methods, and types of resistance. Features sterilization methods, and more. Previous edition: c1999. DNLM: Sterilization--methods. - In addition to citations & references found in this article, see the research citations given at the end of the related articles found at our suggested
CONTINUE READING or RECOMMENDED ARTICLES.
- Carson, Dunlop & Associates Ltd., 120 Carlton Street Suite 407, Toronto ON M5A 4K2. Tel: (416) 964-9415 1-800-268-7070 Email: info@carsondunlop.com. Alan Carson is a past president of ASHI, the American Society of Home Inspectors.
Thanks to Alan Carson and Bob Dunlop, for permission for InspectAPedia to use text excerpts from The HOME REFERENCE BOOK - the Encyclopedia of Homes and to use illustrations from The ILLUSTRATED HOME .
Carson Dunlop Associates provides extensive home inspection education and report writing material. In gratitude we provide links to tsome Carson Dunlop Associates products and services.

