 Water pH Standards & Adjustments
Water pH Standards & Adjustments
Effects, cures for too low pH or too high pH in water
- POST a QUESTION or COMMENT about this article topic.
Recommended pH for drinking water, effects of too high pH or too low pH water - acidic water.
This article provides the WHO guideline for recommended pH for drinking water. We discuss water that is too high in pH and water that is too low in pH. We suggest ways to correct corrosive, aggressive, or too- acidic well water.
This article series describes effects of low pH, acidic or corrosive water on building piping, leaks, dissolved copper, health hazards, and the plumbing system in general. We describe how to detect corrosive or aggressive water and what should be done about it.
InspectAPedia tolerates no conflicts of interest. We have no relationship with advertisers, products, or services discussed at this website.
- Daniel Friedman, Publisher/Editor/Author - See WHO ARE WE?
The World Health Organization, WHO Advice on Water pH - health effects
Article Contents
Effects of Exposure to Extreme pH Values in Water
- Exposure to extreme pH values results in irritation to the eyes, skin, and mucous membranes.
- Eye irritation and exacerbation of skin disorders have been associated with pH values greater than 11. In addition, solutions of pH 10–12.5 have been reported to cause hair fibres to swell (10). In sensitive individuals, gastrointestinal irritation may also occur.
- Exposure to low pH values can also result in similar effects.
- Below pH 4, redness and irritation of the eyes have been reported, the severity of which increases with decreasing pH.
- Below pH 2.5, damage to the epithelium is irreversible and extensive (10).
- In addition, because pH can affect the degree of corrosion of metals as well as disinfection efficiency, it may have an indirect effect on health.
- In addition, if well water at a particular building also happens to require disinfection, the pH is important and needs to be below 8.
What is the Optimum pH for a Building Water Supply?
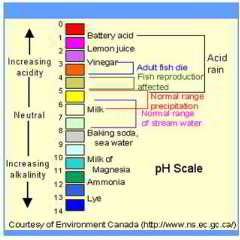
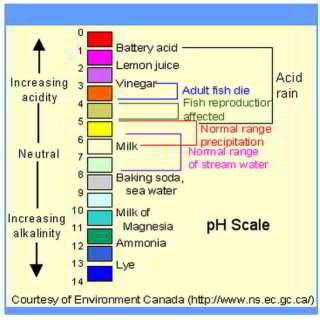
WHO says in general the optimum pH target for drinking water should be 6.5 - 9.5, adding " No health-based guideline value is proposed for pH." [but they didn't appear to consider the effects of dissolved copper - if it occurs]
Acidic Water Treatments to Raise the pH (lower acidity): Calcite Filters vs. Soda Ash Injection
According to the Clean Water Systems & Stores (who sell this equipment) , calcite neutralizer filters
... will typically raise the pH of the water to 7.0 to 8.0 and add 30 to 100 ppm of hardness depending on the alkalinity and water hardness. [2]
More sophisticated is a soda ash injection system. That approach requires a pump and metering device along with an intermediate tank to give the water enough contact time with the soda ash. And room to store 25 or 50 pound bags of soda ash.
Like some other water treatment systems (like a chlorine injector) the soda ash injector pump is hooked up to run when the well pump turns on. It seems that a soda-ash injector system is recommended when the low pH of water is due to dissolved mineral acids (do you live near a mining site or in New York a natural gas from shale mining operation?).
Chemical Feed / Neutralization Soda Ash: water treatment systems dispensing a soda ash chemical feed are used to correct high water pH (acidic) conditions by injecting a base (soda ash) into the water supply followeed by filtration. Water high in natural acids or high in carbon dioxide (CO2) are acidic and are likely to need a treatment of this type.
See WATER TREATMENT EQUIPMENT CHOICES
Acidic Well Water Advice
Reader question: Sage advice needed about corrosive / acidic well water
I have fairly acidic well water on Cape Cod – pH 6.3. I get a lot of blue stains on bathtubs and sinks, which I assume is copper leached from the inside of the copper water-supply piping. I built the house about 16 years ago and replaced the water heater a few years ago.
Am I at risk of developing pin holes or other damage to the copper plumbing and other equipment, including an electric water heater, boiler, and baseboard radiation?
If that’s the case, what’s the preferred (and cheapest) solution: calcite neutralizer tank, soda ash feeder, or other?
FYI, copper level was last measured at 0.11 mg/L, well below the MCL of 1.3, so I don’t think there’s a health issue. But I figure if copper gets leached from the pipes for enough years, at some point there won’t be much pipe left.
Muchas gracias, Steven Bliss, 4 Burlington, VT
Reply:
I do not have experience with pH treatment first hand, but it appears to me that the short answer is that for most situations, calcite filters are the easiest and cheapest method of treatment. But certainly experts have been looking at this problem for along time, and continue to research it. See Mattsson et als [14][15].
Before choosing one I'd measure the LANGLIER SATURATION INDEX (LSI) of the water so you can figure out the level of treatment needed and check that against the treatment method proposed.
Incidentally, before water testing or asking your test company about what they commonly find in wells in your area of Cape Cod, don't rule out the water supply itself as contributing to the supply of dissolved copper.
You could easily distinguish between copper from acidic effects on copper water piping and copper in the water supply by comparing two water tests, one taken from in the building (I give some aggressive testing suggestions below) and one taken from water where it enters the building, presumably through a plastic or galvanized iron well pipe (not a copper one).
Until you know the score, if there is a worry that pipes may already be thin or at risk, that's an extra reason to shut off water when leaving the property unattended.
Some of the suggestions at WINTERIZE A BUILDING might help.
Hot water and water pH - what happens to pH when we heat up the water?
You could collect a hot water sample as well, running water until it's hot, but because the "hot" water has arrived fresh from the water heater tank, it has not sat overnight in the piping and so is not such an aggressive test - I wouldn't bother. Not unless your home uses an antique copper water heater tank.
The WHO guidelines for drinking water quality points out some interesting technical detail,including that (at least for pure water) heating the water will decrease the water's pH. A pH decrease of 0.45 occurs when you raise water temperature by 25 deg. C. [13]
If the house has part plastic water supply piping, you'd want to see where the copper is located, calculate (by linear feet of pipe and its diameter) how much cold to run out to be sure you've collected a sample from inside the copper pipes.
...
Continue reading at LANGLIER SATURATION INDEX LSI or select a topic from the closely-related articles below, or see the complete ARTICLE INDEX.
Or see these
Recommended Articles
- CORROSIVITY or ACIDITY of WATER
- LANGLIER SATURATION INDEX LSI
- OTHER CAUSES of PINHOLE LEAKS in COPPER PIPES
- OTHER CAUSES of PIPE PITTING: pH & HCO3 LEVELS
- PINHOLE LEAKS by ELECTRICAL GROUND ERRORS
- pH of DRINKING WATER, HEALTH EFFECTS
- pH TARGET for DRINKING WATER, WHO
- pH TOO LOW, ACIDIC WATER ADVICE
- pH INCREASE to REDUCE ACIDITY
- WATER TEMPERATURE EFFECT on pH
Suggested citation for this web page
pH of DRINKING WATER, HEALTH EFFECTS at InspectApedia.com - online encyclopedia of building & environmental inspection, testing, diagnosis, repair, & problem prevention advice.
Or see this
INDEX to RELATED ARTICLES: ARTICLE INDEX to WATER TREATMENT SYSTEMS
Or use the SEARCH BOX found below to Ask a Question or Search InspectApedia
Ask a Question or Search InspectApedia
Try the search box just below, or if you prefer, post a question or comment in the Comments box below and we will respond promptly.
Search the InspectApedia website
Note: appearance of your Comment below may be delayed: if your comment contains an image, photograph, web link, or text that looks to the software as if it might be a web link, your posting will appear after it has been approved by a moderator. Apologies for the delay.
Only one image can be added per comment but you can post as many comments, and therefore images, as you like.
You will not receive a notification when a response to your question has been posted.
Please bookmark this page to make it easy for you to check back for our response.
Our Comment Box is provided by Countable Web Productions countable.ca
Citations & References
In addition to any citations in the article above, a full list is available on request.
- De Waard, C., and D. E. Milliams. "Carbonic acid corrosion of steel." Corrosion 31, no. 5 (1975): 177-181.
- Isaac, R. A., L. Gil, A. N. Cooperman, K. Hulme, B. Eddy, M. Ruiz, K. Jacobson, C. Larson, and O. C. Pancorbo. "Corrosion in drinking water distribution systems: a major contributor of copper and lead to wastewaters and effluents." Environmental science & technology 31, no. 11 (1997): 3198-3203.
- Jones, Anne. "Stress corrosion cracking." In in ASM Handbook, Metals Handbook. 1998.
- Kermani, M. B., and A. Morshed. "Carbon dioxide corrosion in oil and gas production—a compendium." Corrosion 59, no. 8 (2003): 659-683.
- Kiene, L., W. Lu, and Y. Levi. "Relative importance of the phenomena responsible for chlorine decay in drinking water distribution systems." Water Science and Technology 38, no. 6 (1998): 219-227.
- Kritzer, Peter. "Corrosion in high-temperature and supercritical water and aqueous solutions: a review." The Journal of Supercritical Fluids 29, no. 1 (2004): 1-29.
- Little, Brenda J., Florian B. Mansfeld, Peggy J. Arps, and James C. Earthman. Microbiologically influenced corrosion. Wiley‐VCH Verlag GmbH & Co. KGaA, 2007.
- Oram, Brian, "Drinking Water Issues Corrosive Water (Lead, Copper, Aluminum, Zinc, and More)" (web page), Water Research Center, Water Research Center B.F. Environmental Consultants Inc. 15 Hillcrest Drive, Dallas, PA 18612, USA, retrieved 2017/02/16, original source: http://www.water-research.net/index.php/drinking-water-issues-corrosive-water-lead-copper-aluminum-zinc-and-more
- Roberge, P. R. (1999). Handbook of Corrosion Engineering (1st ed.). McGraw-Hill Professional. ISBN 0-07-076516-2.
- Sigler, W. Adam, and Bauder, Jim, "CORROSIVITY" [PDF], Montana State University Extension, Water Quality Program, Department of Land Resources and Environmental Sciences, (un-dated) retrieved 2017/02/16, original source: http://waterquality.montana.edu/well-ed/files-images/Corrosivity.pdf - note that this page was adapted from Wilkes University Center For Environmental Quality; Corrosion, Saturation Index, Balanced Water in Drinking Water Systems
- Speller, Corrosion. Causes and Prevention of Corrosion in Steam and Hot Water Heating Systems. McGraw-Hill, 1951.
- Volk, Christian, Esther Dundore, John Schiermann, and Mark LeChevallier. "Practical evaluation of iron corrosion control in a drinking water distribution system." Water research 34, no. 6 (2000): 1967-1974.
- Was, G. S., P. Ampornrat, G. Gupta, S. Teysseyre, E. A. West, T. R. Allen, K. Sridharan et al. "Corrosion and stress corrosion cracking in supercritical water." Journal of Nuclear Materials 371, no. 1 (2007): 176-201.
- [2] Clean Water Systems & Stores, Inc. 2806-A Soquel Ave, Santa Cruz, California 95062, Telephone: 1-888-600-5426 or international: 1-831-462-8500 . web search 4/23/12, original source: - cleanwaterstore.com/copper-pipe-corrosion.html
- [4] "Langelier Saturation Index (LSI), Wikipedia Web: https://www.wikipedia.org/ provided background information about some topics discussed at this website provided this citation is also found in the same article along with a " retrieved on" date. NOTE: because Wikipedia entries are fluid and can be amended in real time, we cite the retrieval date of Wikipedia citations and we do not assert that the information found there is necessarily authoritative.
- [5] "Drinking Water Contaminants, List of Contaminants & their MCLs", U.S. EPA United States Environmental Protection Agency, National Primary Drinking Water Regulations, web search 4/23/12, original source: http://water.epa.gov/drink/contaminants/index.cfm#List
- [6] "Basic Information about Copper in Drinking Water", U.S. EPA United States Environmental Protection Agency, web search 4/23/12, original source: http://water.epa.gov/drink/contaminants/basicinformation/copper.cfm
- [7] "Fin Tube / Bare Elements", Slant/Fin Boilers & Baseboards, Slant/Fin Corporation, 100 Forest Drive, Greenvale, NY 11548, Phone: (516) 484-2600, Fax: (516) 484-5921, E-mail: info@slantfin.com, web search 4/23/12, original source: http://www.slantfin.com/index.php/products/baseboard-residential/fin-tube--bare-elements
- ABS Plastic Drain/Waste/Vent (DWV) pipe failures: reported for Centaur, Phoenix, Polaris, Gable, and Spartan pipe mfgs. for pipe made between 1985 and 1988. CPSC Hot Line: 800-638-8270 or ABS Drain Leaks/Failures-Class Action Settlement COX settlement through Shell Oil set up by a contractor involved in the settlement. Polybutylene Plumbing Failures: Spencer Class settlement, Web: spencerclass.com, 10% of replacement cost/damages, only for acetal (plastic)fittings Polybutylene plumbing lawsuit proposed settlement-old site
- [13] "Guidelines for drinking-water quality", 2nd ed. Vol. 2. Health criteria and other supporting information. World Health Organization, Geneva, 1996. WHO, op.cit.
- [14] "Pitting Corrosion in Copper Tubes – Cause of Corrosion and Counter-Measures", Mattsson, E.; Fredriksson, A.-M., British Corrosion Journal, Volume 3, Number 5, September 1968 , pp. 246-257(12), Maney Publishing, Quoting the article abstract:
An investigation of failures of hard-drawn copper water pipes (phosphorus-deoxidised copper) in service due to pitting corrosion was conducted from November, 1962 to February, 1965. Fifteen cases were reported. All those about which information could be obtained came from hot water installations and occurred in water with a low pH (?7) and a HCO3- content of, at the most, 100 mg/l but generally below 50 mg/1. Failures not due to pitting corrosion (i.e. caused by erosion and corrosion or corrosion fatigue) occurred in waters with a higher pH and higher HCO3- content.
A laboratory investigation into the ability of the corrosion products to counteract further corrosion in different types of water was also carried out, using an electrolytic cell which, in principle, was a model of an active pit in a copper tube. This led to the following conclusions, which are in good agreement with the results obtained from the examination of service failures:
If the pH value of the water is high enough, the copper dissolved by the corrosion can be precipitated as basic copper salt. At low pH values such precipitation does not take place.
If the [HCO3?]/[SO42?] ratio in the water is high, dissolved copper can be precipitated as basic copper carbonate in the neighbourhood of the corrosion site and counteract further corrosion.
At a low [HCO3?]/[SO42?] ratio, crusts of basic copper sulphate will be precipitated at some distance from the corrosion site and may lead to a high corrosion rate.
Pitting is not likely to occur in hot water tubes of hard copper if the pH is ? 7·4 and the [HCO3?]/[SO42?] ratio ?1 (the concentrations given in mg/1). The critical values mentioned are approximate and may be adjusted in the light of future experience. - [15] "Health and aesthetic impacts of copper corrosion on drinking water",
Dietrich AM, Glindemann D, Pizarro F, Gidi V, Olivares M, Araya M, Camper A, Duncan S, Dwyer S, Whelton AJ, Younos T, Subramanian S, Burlingame GA, Khiari D, Edwards M., Virginia Tech, Blacksburg, VA 24061-0246, USA. andread@vt.edu, Water Sci Technol. 2004;49(2):55-62., Abstract
Traditional research has focused on the visible effects of corrosion--failures, leaks, and financial debits--and often overlooked the more hidden health and aesthetic aspects. Clearly, corrosion of copper pipe can lead to levels of copper in the drinking water that exceed health guidelines and cause bitter or metallic tasting water. Because water will continue to be conveyed to consumers worldwide through metal pipes, the water industry has to consider both the effects of water quality on corrosion and the effects of corrosion on water quality. Integrating four key factors--chemical/biological causes, economics, health and aesthetics--is critical for managing the distribution system to produce safe water that consumers will use with confidence. As technological developments improve copper pipes to minimize scaling and corrosion, it is essential to consider the health and aesthetic effects on an equal plane with chemical/biological causes and economics to produce water that is acceptable for public consumption. - Our recommended books about building & mechanical systems design, inspection, problem diagnosis, and repair, and about indoor environment and IAQ testing, diagnosis, and cleanup are at the InspectAPedia Bookstore. Also see our Book Reviews - InspectAPedia.
- In addition to citations & references found in this article, see the research citations given at the end of the related articles found at our suggested
CONTINUE READING or RECOMMENDED ARTICLES.
- Carson, Dunlop & Associates Ltd., 120 Carlton Street Suite 407, Toronto ON M5A 4K2. Tel: (416) 964-9415 1-800-268-7070 Email: info@carsondunlop.com. Alan Carson is a past president of ASHI, the American Society of Home Inspectors.
Thanks to Alan Carson and Bob Dunlop, for permission for InspectAPedia to use text excerpts from The HOME REFERENCE BOOK - the Encyclopedia of Homes and to use illustrations from The ILLUSTRATED HOME .
Carson Dunlop Associates provides extensive home inspection education and report writing material. In gratitude we provide links to tsome Carson Dunlop Associates products and services.

