Carbon Monoxide Gas Guide
Carbon Monoxide Gas Guide
CO
Exposure, Carbon Monoxide CO Poisoning Symptoms, Carbon Monoxide Exposure Limits, Cause of Death, Chronic Exposure Effects, CO Gas Testing Procedures
- POST a QUESTION or COMMENT about carbon monoxide gas exposure, hazards, testing, and regulations
Carbon monoxide (CO) gas hazards home page: carbon monoxide alarms, effects, tests, exposure limits, medical effects.
This article describes the toxicity and exposure limits for exposure to carbon monoxide gas (CO).
In companion articles we also describe procedures for inspecting buildings for evidence of carbon monoxide hazards, including visual clues that may suggest a safety problem even if CO testing is not detecting carbon monoxide at the time of the inspection.
We describe carbon monoxide testing methods, the medical effects of carbon monoxide exposure, and we cite authorities who set the limits on carbon monoxide exposure in different environments.
We give references and explanation regarding Toxicity of Carbon Monoxide, based on literature search and including research on OSHA, NIOSH, and at the old Compuserve's Safety Forum.
This is background information, obtained from expert sources. This text may assist readers in understanding these topics.
InspectAPedia tolerates no conflicts of interest. We have no relationship with advertisers, products, or services discussed at this website.
- Daniel Friedman, Publisher/Editor/Author - See WHO ARE WE?
Guide to Inspecting buildings for Visible Evidence of Conditions Likely to Produce Dangerous Carbon Monoxide Gas
Discussed here: Guide to Inspecting buildings for Visible Evidence of Conditions Likely to Produce Dangerous Carbon Monoxide Gas,
IF YOU SUSPECT CARBON MONOXIDE POISONING GO INTO FRESH AIR IMMEDIATELY and get others out of the building, then call your fire department or emergency services for help. Links on this page also direct the reader to carbon dioxide gas information in a separate document.
Seek prompt advice from your doctor or health/safety experts if you have any reason to be concerned about exposure to toxic gases. Carbon monoxide poisoning can be fatal but exposure at lower limits can produce flu-like symptoms and headaches that are often mistaken for ordinary illness.
The fact that you cannot see nor smell dangerous carbon monoxide gas does not mean that there is nothing to look for when assessing the safety of heating equipment. Not only are there easily spotted installation errors (the first list below), there may be more subtle but easily visible errors if you know what to look for (the second list below).
Safety Suggestions: Install Carbon Monoxide Detectors in addition to Smoke Detectors
Carbon monoxide detectors are inexpensive and readily available, both as a battery-operated unit and as a unit that plugs into an electrical outlet in the home. No home should be without this safety protection, and homes with gas-fired equipment (natural gas or LP propane), space heaters, or other sources of risk should be extra cautious. Smoke detectors do not protect against carbon monoxide poisoning, and the opposite is also true. Carbon monoxide detectors do not warn of smoke or fire.
Examples of visible building conditions risking carbon monoxide hazards
This is by no means the complete list of errors that can cause dangerous carbon monoxide exposure in buildings, but here are some common foul ups outside of the workplace that can cause dangerous levels of indoor carbon monoxide:
- Space heaters: improper use of gas or kerosene fired heaters can produce high indoor CO levels. Warning: Never go to sleep in an enclosed space with a space heater left operating. In addition to the CO hazards there is a risk of oxygen depletion which can also lead to asphyxiation.
- Gas fired central heating equipment combined with:
- Improper venting, blocked, under-sized, over-sized, missing parts, improperly sloped chimney or flue. A variety of errors can cause a failure to vent combustion gases out of the building, allowing dangerous flue gases to build up indoors.
- Inadequate combustion air. If a heating appliance is installed in a small confined space it must be provided with outside combustion air. A service technician may tune and inspect a gas-fired boiler with the boiler room door open, finding that it seems to operate fine. When s/he closes it on leaving, there may be an inadequate or no opening for combustion air into the room.
- Venting small appliances into large cold chimneys: Installation of small, higher efficiency gas-fired equipment into old homes at which the appliance is vented into a large (cold) masonry chimney. In such instances the heater may never develop sufficient heat and draft to actually vent up the chimney.
- Also sometimes water heaters are left venting into a too-large, too-cold masonry chimney after a gas-fired boiler is converted to a high-efficiency direct-vent (no chimney) unit. One of my clients developed headaches every October - an event I traced to this condition in Poughkeepsie, NY. [DF re E.B. case 1988].
- Car exhaust, such as to occupants of rooms adjoining or even above a garage where car engines are left running
- Un-vented gas fired water heaters, often found venting directly into a basement utility room or even directly into a living area or bedroom.
- CHIMNEY INSPECTION GUIDE contains detailed suggestions for inspecting building chimneys including the detection of blocked chimney flues or indications that a chimney may be blocked.
- Exhaust leaks in vehicles -
see BOAT & CAR SMELLS & ODORS; car exhaust can leak into the vehicle itself, or if a car, camper, or truck engine is left running in a building garage, the vehicle exhaust can leak into the building at dangerous levels.
Other clues which can suggest a risk of carbon monoxide hazards in buildings
- CO detector alarms Do not ignore this first line of defense. Install CO detectors near the heating equipment as well as in sleeping areas of the home. People have died after not believing their CO detector and taking out the batter to silence the annoying device which they believed was malfunctioning.
- Missing HVACR equipment parts: Gas fired water heaters, furnaces, boilers which are missing flue vent connector components such as draft hoods and flue gas spill detection switches - it can be difficult to spot that something is missing unless you know what's supposed to be there. Review this topic with a trained heating service technician or plumber.
- Clogged heater draft hood from hair or other debris
- Signs of flue gas spillage Blocked flues will result in combustion gas spillage back into the building. Often this will cause:
- Rust on heating equipment at the point of flue gas spillage - you can detect this even when the equipment is not operating
- Rusty debris on the top of gas fired heating equipment below the draft hood
- Water condensation on building surfaces may occur if gas-vented appliances are venting back into the building, especially on cool basement surfaces - you can only observe this when the equipment is operating
- Odors of combustion products: while CO and CO2 are themselves odorless, if they are spilling from heating equipment, odors of other combustion products may be notices
- Soot visible at the raft hood or below or on the burner at gas fired equipment
Procedures for Testing for Carbon Monoxide Gas Leaks, Exposure, Hazards
In addition to the installation of CO monitoring alarms in buildings, a variety of electronic and gas sampling equipment is available to make spot checks for hazardous gases. I have and have used a variety of these devices under a wide range of conditions.
While a "positive" indication of a gas is an important indicator of a hazard, a "negative" or "not found" result is nothing to rely on.
The fact that dangerous levels of CO are not present in a building at a particular instant is absolutely no guarantee that dangerous levels of CO (for example) may not occur even moments later. For example, opening a window, turning on a fan or clothes dryer, closing a door, and similar innocent acts can significantly change air flow, combustion air, and other building conditions.
Therefore spot tests for dangerous gases should not be relied upon to guarantee building safety. This is why the list of visual inspection items and proper heating equipment maintenance are so important.
Medical effects of Carbon Monoxide (CO) Poisoning
Watch out: the immediate and most severe effect of carbon monoxide exposure and poisoning can be death, typically loss of consciousness followed by death. In a recent example of carbon monoxide fatality, the manager of a Legal Seafoods restaurant in Huntington Long Island (New York) died and twenty-seven others were sickened as a result of carbon monoxide poisoning traced to a faulty water heating equipment vent.
News media noted that while CO detectors are required in homes just about everywhere, laws requiring carbon monoxide detectors in the workplace are not (as of February 2014) in place in New York where this death occurred.
The LA Times news report illustrates the pernicious odorless nature of carbon monoxide gas: when restaurant workers reported that the assistant-manager was unconscious, apparently from a head injury due to a fall, rescue workers, themselves sickened when working in the building, found the restaurant manager also unconscious, but he was later pronounced from carbon monoxide poisoning. - Los Angeles Times (2014)
Here we discuss the effects of CO exposure or chronic CO exposure at less severe levels when death, the ultimate penalty, has not been incurred.
Many sources I (DF) reviewed indicated that if carbon monoxide exposure was sub-acute, that is if the person did not lose consciousness and was removed from the CO exposure before losing consciousness, then any medical effects were temporary. Indeed detection of CO exposure at a hospital is problematic since CO leaves the bloodstream quickly once a person is exposed to normal air.
However there is evidence that lasting physical damage may occur from carbon monoxide exposure, though the popular press has not (2006) discussed the exposure level and duration necessary for these effects.
Heart muscle damage occurs from Carbon Monoxide (CO) exposure, screening recommended
31 January 2006 - The New York Times Science Section reports on a new study, released in JAMA's January 25 2006 Magazine Issue, and which indicated that people exposed to carbon monoxide suffer damage to their heart muscles and are at much greater risk for heart attacks in later years.
The Times article asserted that CO Poisoning results in 40,000 emergency visits a year in the United States - the most common accidental poisoning event in the U.S. with an annual average accidental death rate of about 1000 people and average suicidal death rate of about 2400 people. [U.S. CDC] Five percent of such patients die in the hospital. Research was not cited regarding sub acute exposures and exposures which do not result in a visit to a hospital. -- New York Times Science Section, January 31, 2006 p. F6, "After Crisis, Carbon Monoxide Still Takes a Toll."
The carbon monoxide exposure and heart muscle damage study was led by Christopher R. Henry, Minneapolis Heart Institute Foundation, in the current [Jan 2006] Journal of the American Medical Association The study examined the medical history of 230 people exposed to carbon monoxide and treated at hospital between 1994 and 2002, following their health to 2005.
After 7 1/2 years, in this otherwise low risk (of heart failure) population, 25% of the originally-surviving patients had died - a rate about three times the average heart failure death rate statistic. For people who had suffered heart muscle damage the mortality rate was 38% with half of the mortalities being (apparently) traced to cardiovascular problems.
The study concludes that people who are exposed to carbon monoxide should be screened for heart muscle damage. Heart muscle damage from CO poisoning (in the study) was characterized by elevated levels of cardiac troponin I (a type of protein) or creatine kinase-MB (a type of enzyme), and/or changes in diagnostic electrocardiogram (ECG). -- DJ Friedman paraphrasing the NY Times article and JAMA's news release regarding this study.
More references for this study:
see: Heart Injury Due to Carbon Monoxide Poisoning Increases Long-Term Risk of Death, JAMA January 25 2006. AMA news release 2006. This study was supported by an unrestricted educational grant from the Minneapolis Heart Institute Foundation. Study copies may be available from the JAMA/Archives Media Relations Department at 312/464-JAMA (5262) - mediarelations@jama-archives.org.
Carbon Monoxide Poisoning Information From U.S. Army Field Manual 8-285:
Pathology of CO poisoning: Asphyxiation is caused by the inactivation of blood hemoglobin through a combination with CO. The resultant anoxia may produce nervous system changes. Postmortem examinations reveal little beyond the characteristic cherry red color of the blood and hemorrhages in the brain
Symptoms of CO poisoning: Carbon monoxide is very insidious in its action and poisoning may occur without appreciable initial signs. The symptoms progress from throbbing headaches, vertigo, yawning, and poor visual acuity, to the development of cherry red mucous membranes, weakness and coma, subnormal temperature, feeble pulse, and death.
Diagnosis of CO poisoning: The diagnosis is made from the
circumstances of exposure and the appearance of
cherry red skin and mucous membranes color.
Protection against CO poisoning: In general, exposure to CO should be avoided whenever possible.
Adequate ventilation
should be provided for all enclosed spaces where CO
may be produced. The safety of air in the space for
people to breathe may be tested by standard CO indicator or detector devices. Individuals required to
enter closed areas where high concentrations of CO
are (known or suspected to be) present must be
provided with respiratory protective devices. For the
approved devices, refer to TB MED 502.
Note: For a helpful discussion of just what constitutes "adequate venitlation", see Offermann, Francis (Bud) J., P.E., C.I.H., ASHRAE & Mark Nicas, Ph.D., MPH, C.I.H.,
USE WITH ADEQUATE VENTILATION ? [PDF], ASHRAE Journal, May 2018.
Treatment for Carbon Monoxide Poisoning:
Remove the victim to pure air. If respirations are weak or absent, begin assisted ventilation at once. Oxygen, if available, should be given by a face mask, preferably under pressure (up to 3 atmospheres). The patient should be kept warm and at rest (sedated, if necessary).
After resuscitation, initial supportive measures (such as the need for parenteral fluids and pressor drugs) can best be decided by the medical officer. Ordinarily, methylene blue solution, morphine, and atropine should NOT be used (TB MED 269).
Prognosis for people exposed to carbon monoxide poisoning:
The longer the period of the coma, the less the chance for recovery. Most mildly exposed individuals recover with early treatment. Tachycardia and dyspnea may continue for months. There may be CNS disturbances ranging from simple neuritis to mental deterioration.
CO EXPOSURE LIMITS - Carbon monoxide exposure limits PEL and TLV set by OSHA and NIOSH
This section has been moved to CO EXPOSURE LIMITS.
To convert between % and ppm concentration of carbon monoxide air
see CONVERT PPM to % CONCENTRATION
Carbon Monoxide Hazards - Carboxyhemoglobin levels and related health effects on people
Quoting from CPSC Indoor Air Pollution Book Online Copy
Carbon monoxide is an asphyxiant. An accumulation of this odorless, colorless gas may result in a varied constellation of symptoms deriving from the compound's affinity for and combination with hemoglobin, forming carboxyhemoglobin (COHb) and disrupting oxygen transport. The elderly, the fetus, and persons with cardiovascular and pulmonary diseases are particularly sensitive to elevated CO levels.
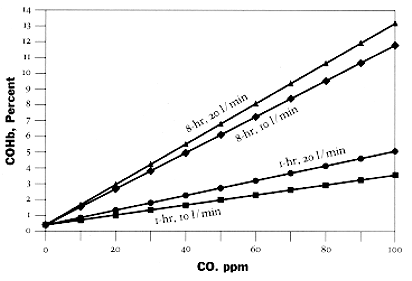
Methylene chloride, found in some common household products, such as paint strippers, can be metabolized to form carbon monoxide which combines with hemoglobin to form COHb. The following chart shows the relationship between CO concentrations and COHb levels in blood.
Tissues with the highest oxygen needs -- myocardium, brain, and exercising muscle -- are the first affected. Symptoms may mimic influenza and include fatigue, headache, dizziness, nausea and vomiting, cognitive impairment, and tachycardia. Retinal hemorrhage on funduscopic examination is an important diagnostic sign19, but COHb must be present before this finding can be made, and the diagnosis is not exclusive.
Studies involving controlled exposure have also shown that CO exposure shortens time to the onset of angina in exercising individuals with ischemic heart disease and decreases exercise tolerance in those with chronic obstructive pulmonary disease (COPD)20.
Note: Since CO poisoning can mimic influenza, the health care provider should be suspicious when an entire family exhibits such symptoms at the start of the heating season and symptoms persist with medical treatment and time.
Relationship between
carbon monoxide (CO) concentrations and carboxyhemoglobin (COHb) levels in blood
Predicted COHb levels resulting from 1- and
8-hour exposures to carbon monoxide at rest (10 l/min) and with light
exercise (20 l/min) are based on the Coburn-Foster-Kane equation using the following
assumed parameters for nonsmoking adults: altitude = 0 ft; initial COHb level = 0.5%; Haldane constant = 218; blood volume =
5.5 l; hemoglobin level = 15 g/100ml; lung diffusivity = 30 ml/torr/min; endogenous rate = 0.007 ml/min.
Source: Raub, J.A. and Grant, L.D. 1989.
"Critical health issues associated with review of the scientific
criteria for carbon monoxide." Presented at the 82nd Annual Meeting of
the Air Waste Management Association. June 25-30.
Anaheim, CA. Paper No. 89.54.1, Used with
permission.
Table of Carboxyhemoglobin levels and related health effects on people
% COHb in blood |
Effects Associated with this COHb Level |
80 |
Deatha |
60 |
Loss of consciousness; death if exposure continuesa |
40 |
Confusion; collapse on exercisea |
30 |
Headache; fatigue; impaired judgmenta |
7-20 |
Statistically significant decreased maximal oxygen consumption during strenuous exercise in healthy young menb |
5-17 |
Statistically significant diminution of visual perception, manual dexterity, ability to learn, or performance in complex sensorimotor tasks (such as driving)b |
5-5.5 |
Statistically significant decreased maximal oxygen consumption and exercise time during strenuous exercise in young healthy menb |
Below 5 |
No statistically significant vigilance decrements after exposure to COb |
2.9-4.5 |
Statistically significant decreased exercise capacity (i.e., shortened duration of exercise before onset of pain) in patients with angina pectoris and increased duration of angina attacksb |
2.3-4.3 |
Statistically significant decreased (about 3-7%) work time to exhaustion in exercising healthy menb |
- SOURCE: a U.S. EPA (1979); b U.S. EPA (1985)
Nitrogen dioxide (NO) and sulfur dioxide (SO2) act mainly as irritants, affecting the mucosa of the eyes, nose, throat, and respiratory tract. Acute S02-related bronchial constriction may also occur in people with asthma or as a hypersensitivity reaction. Extremely high-dose exposure (as in a building fire) to N02 may result in pulmonary edema and diffuse lung injury. Continued exposure to high N02 levels can contribute to the development of acute or chronic bronchitis.
The relatively low water solubility of N02 results in minimal mucous membrane irritation of the upper airway. The principal site of toxicity is the lower respiratory tract. Recent studies indicate that low-level N02 exposure may cause increased bronchial reactivity in some asthmatics, decreased lung function in patients with chronic obstructive pulmonary disease, and an increased risk of respiratory infections, especially in young children.
The high water solubility of S02 causes it to be extremely irritating to the eyes and upper respiratory tract. Concentrations above six parts per million produce mucous membrane irritation. Epidemiologic studies indicate that chronic exposure to S02 is associated with increased respiratory symptoms and decrements in pulmonary function21. Clinical studies have found that some asthmatics respond with bronchoconstriction to even brief exposure to S02 levels as low as 0.4 parts per million22
...
Reader Comments, Questions & Answers About The Article Above
Below you will find questions and answers previously posted on this page at its page bottom reader comment box.
Reader Q&A - also see RECOMMENDED ARTICLES & FAQs
Question: My furnace service tech shut down my gas fired unit and says I need a new one. He said he could smell the CO and he wouldn't let me see his CO detector meter. Am I getting scammed?
I recently had a serviceman come to work on our natural gas furnace. When he was done he said that it was unsafe and needed to be replaced. He indicated that there was a high level of carbon monoxide (10,000 ppm) and shut the furnace off.
I was made a little suspicious when he directed me to the exhaust pipe for the furnace and told me that the leak was so bad he could smell the CO. Since I always thought that CO is odourless that hit me a little strange. Plus the fact that he shoved the meter he was using under my nose for 3 seconds, not enough time for me to even read what was on the display.
His sales pitch was that the heat exchanged was clogged and needed to be replaced. Would there really be a CO level that high with a blocked exchanger or would it need to have a leak? Is it possible he is just trying to get some work out of me and hoping that I won't know any better? - Matthew
Reply: CO itself is odorless, but mixed in with combustion flue gases there would be a "flue gas" smell that someone might observe;
Watch out: Because of very serious life safety issues, do not turn the equipment back on.
But before replacing the gas furnace due to a carbon monoxide hazard I'd want an accurate diagnosis of just what the problem really is. Some problems might indeed justify a whole new heater, for example if the heat exchanger is perforated and if the cost to remove and replace the damaged heat exchanger is close to the cost of a whole new furnace, I'd consider going with the latter.
Other problems can cause flue gas spillage, equally dangerous, but with different solutions such as
- A blocked chimney
- Inadequate combustion air
- Improperly adjusted gas burners and air shutters
I wouldn't insult the service tech when s/he may have been saving your life. But it's quite reasonable to ask that a carbon monoxide hazard be properly diagnosed and the evidence shown to you before you approve equipment replacement. After all, if you replace the gas furnace but leave an unsafe chimney in place a fatal carbon monoxide hazard could still be present.
Question: what does a utility company gas furnace safety check red-tag placed on our heating equipment mean?
When your Utility Company Technician performs a safety check of your gas furnace and determines it to be "unsafe/hazardous" plus "Shut off and capped due to recirculation," what exactly does this mean? - Chris
Reply: significance of red-tagging heating equipment - serious safety hazards are present and the equipment cannot be safely used until repaired
Chris: details may vary by state or municipality, but in general you are describing what the industry calls "red tagging" - a utility company or a heating contractor or even a licensed plumber or other trades expert who inspects heating equipment and finds that it is in a condition that is unsafe to operate should leave the system turned off and red-tagged with - usually - an attached red safety tag that indicates the date, the identity of the inspector, and the unsafe condition.
Red-tagged equipment should not be turned back on before it has been repaired and confirmed safe to operate.
There are some wrinkles in this scheme however. I've found some oil service companies who put a red border on ALL of their service tags - some lawyer must have decided that that would somehow protect the oil company from a lawsuit should a future unsafe condition be discovered, since the lawyer could argue that the system had already been "red tagged".
That's a boy-who-cried-wolf argument and a sloppy defense that interferes with true unsafe equipment notifications - a bad practice.
So presuming that your equipment was actually found unsafe, and red-tagged, it should indeed be left off until it's repaired. The problem that needs to be fixed is not necessarily expensive - it depends on just what's wrong. But leave your gas furnace off until you know the specifics and they have been addressed.
Question: asphyxiation hazards from gas fired water heaters?
what asphyxiation statistics are available for water heaters and water heater venting? - Dan Lewis
Reply:
Dan that's an interesting way to put a carbon monoxide hazard question. Asphyxiation from CO depends on the concentration and exposure time, as we've published. One could calculate some theoretical carbon monoxide production rates from water heaters of various BTUH input ratings, but frankly in my opinion such data would be wild speculation. That's because the accumulation of a toxic gas in a building or any enclosed space depends on so many variables, such as:
- the actual equipment firing rate and combustion air supply; variations in combustion air supply would vary the CO production rate
- variations in chimney and flue gas venting, depending on indoor temperature, outdoor temperature, heating appliance temperature, appliance run time, lack of combustion air, and chimney design details such as routing, height, horizontal flue vent connector length, impedence of draft regulators or draft hoods, etc.
In sum, I am doubtful that a simple CO output number for a water heater would be credible, and a maximum potential CO output number would fail to consider the many peculiar variables of the building itself.
If you have a more specific case in mind we could try to work out more details.
Question: how to conduct a carbon monoxide CO survey of CO from street traffic?
I live on a residential street in the community of Glendale Ca. My street happens to be the only access to a regional park that was developed by the city. There are not only the hundreds of cars that access the park but also continual city large truck traffic and school busses that are creating high levels of CO. How can I get someone to take readings and correct this situation - Ken Steele
Reply:
Ken,
Because of the high cost of hiring an expert to make CO measurements, and because any individual measurement made at one location at one time is absolutely unreliable as an overall characterization of the CO exposure in or around your home, you'd want measurements that include, as a study the CO levels atdifferent times of day and in different seasons
- at different locations outside and inside the building at specifically documented distances and locations relative to the suspected source (say the center of the street or park entry)
- along with documentation of the level and type of traffic nearby at the time
- Along with baseline measurements of CO levels at other locations that offer points of comparison with your own street or home
All of that data, collected and prepared with sufficient professionalism to be credible would be very expensive, surely thousands of dollars.
A plausible alternative would be to purchase a CO level monitoring instrument, become educated in its proper use, and then to conduct your own less formal study.
Question: what might be causing a smell in my basement?
aerosol smell in basement - what could be the cause - 6/26/2012 Anonymous
Reply:
Gee, Anon, I have no idea what an "aerosol" smell is. But you've posted the question on a carbon monoxide article - CO gas is odorless, though it can appear transported by gases from an oil or gas burner whose other combustion products may smell. For such a broad question, I suggest starting odor track-down
Question: gas odors in my heating system - no leak was detected, why does it still smell
I smell gas in my heating system.. I called for them to do a test... they said no alarm regeisterd a leak..
why is it I still smell gas.. I feel like I am dying from the smell.. can somone please help me?
e-mail me at Biidagress@aol.com
my life depends on it
thank you
JessieReply:
Jessie,
CO alarms and detectors respond to that gas - Carbon Monoxide, but not necessarily to other combustion gases that would be present in escaping heating system exhaust. So if your chimney or heater is leaking - still potentiallyi dangerous including as a possible fire or as a possible inadequate draft equipment problem - relying on a CO detector alone would be nonsense.
Ask your heating service manager to send an experienced technician to inspect your heating equipment for safe, proper operation.
...
Continue reading at CO DETECTION OPTIONS or select a topic from the closely-related articles below, or see the complete ARTICLE INDEX.
Or see these
Recommended Articles
- BACKDRAFTING HEATING EQUIPMENT
- CARBON DIOXIDE - CO2 - a different gas
- CARBON MONOXIDE - CO
- CARBON MONOXIDE WARNINGS: HOME HEATERS
- FEAR-O-METER: Dan's 3 D's SET REPAIR PRIORITIES
- GAS DETECTION INSTRUMENTS
- GAS EXPOSURE EFFECTS, TOXIC
Suggested citation for this web page
CARBON MONOXIDE - CO at InspectApedia.com - online encyclopedia of building & environmental inspection, testing, diagnosis, repair, & problem prevention advice.
Or see this
INDEX to RELATED ARTICLES: ARTICLE INDEX to GAS HAZARDS in BUILDINGS
Or use the SEARCH BOX found below to Ask a Question or Search InspectApedia
Ask a Question or Search InspectApedia
Try the search box just below, or if you prefer, post a question or comment in the Comments box below and we will respond promptly.
Search the InspectApedia website
Note: appearance of your Comment below may be delayed: if your comment contains an image, photograph, web link, or text that looks to the software as if it might be a web link, your posting will appear after it has been approved by a moderator. Apologies for the delay.
Only one image can be added per comment but you can post as many comments, and therefore images, as you like.
You will not receive a notification when a response to your question has been posted.
Please bookmark this page to make it easy for you to check back for our response.
IF above you see "Comment Form is loading comments..." then COMMENT BOX - countable.ca / bawkbox.com IS NOT WORKING.
In any case you are welcome to send an email directly to us at InspectApedia.com at editor@inspectApedia.com
We'll reply to you directly. Please help us help you by noting, in your email, the URL of the InspectApedia page where you wanted to comment.
Citations & References
In addition to any citations in the article above, a full list is available on request.
- Jennifer Moore, Sales Administrator, Nextteq, LLC, Tampa FL, Nexteq 813-249-5888. Nextteq is the master Distributor for Gastec in the United States. According to the company's website, Gastec Gas Sampling Pumps are the industry’s first and only pumps to provide on-the-spot measurement of ambient temperature. [Private email, JM to DF 5/23/08]
- "Choosing and Using a Carbon Monoxide CO Monitor," Dan Friedman, The ASHI Technical Journal, Vol. 2 No. 1, July1991
- Dräger MSDS for Dräger CH25301 Air Current Tubes, Page 1 of 2.
Dräger MSDS for Dräger CH25301 Air Current Tubes , Page 2 of 2.
Watch out: the Dräger air current tube or "smoke tube" # CH16631 produces a sulfuric acid gas sulfuric acid H2SO4 /SO3 that is dangerous to life and is highly corrosive. Take a look at our copy of the Dräger MSDS for their CH25301 Air Current Tubes. We stored this MSDS in the box with the rubber bulb and tube cutter provided by Dräger. These air current monitoring tubes are provided with rubber caps so that the tube can be "stopped" or shut down when not in use. But the sulfuric acid was so corrosive that it not only caused the rubbger caps to disintegrate, it actually "burned" or oxidized our copy of the MSDS paper form! - U.S. Army Field Manual FM-8-285-Noxious_Chemicals discusses Ammonia, Carbon Monoxide, Hydrogen Sulfide, Oxides of Nitrogen, Hazards caused by fire,
- Matt Pearce, "Leaky pipe blamed for carbon monoxide death at Long Island restaurant -
Investigators say the restaurant had no carbon monoxide detectors", Los Angeles Times, Nation (online), 2/23/2014, retrieved 2/26/14, original source http://www.latimes.com/nation/la-na-ny-carbon-monoxide-20140224,0,6557080.story#ixzz2uRigtEYb
This Excerpt from the LA Times illustrates comments made our article above: Police and firefighters responded to a Legal Sea Foods restaurant in Huntington, N.Y., on Saturday evening after a report that an assistant manager had fallen and hit her head in the basement. Shortly after arriving, police said, the emergency responders began to feel sick and suspected they had been poisoned by carbon monoxide — a colorless, odorless gas released when something is burning. The gas can cause suffocation. Authorities managed to rescue the assistant manager, but it was too late to save the manager, Steven Nelson, 55, of Copiague, who also was found unconscious in the basement. He was pronounced dead at a hospital. ... The Legal Sea Foods chain promises stronger safety measures ... Such devices are required for New York residences with fuel-burning energy sources or attached garages under "Amanda's Law." The legislation was enacted in 2010 after the death of Amanda Hansen, 16, of West Seneca, who died of carbon monoxide poisoning during a 2009 sleepover at a friend's house." - Air Pollution Toxicology: APTI Course SI:300, Introduction to Air Pollution Toxicology, US EPA Air Pollution Training Institute, Environmental Research Center, Research Triangle Park, NC 27711, Sept. 1993, web search 08/28/2010, original source: http://yosemite.epa.gov/ - Excerpt from the article: Police and firefighters responded to a Legal Sea Foods restaurant in Huntington, N.Y., on Saturday evening after a report that an assistant manager had fallen and hit her head in the basement. Shortly after arriving, police said, the emergency responders began to feel sick and suspected they had been poisoned by carbon monoxide — a colorless, odorless gas released when something is burning. The gas can cause suffocation. Authorities managed to rescue the assistant manager, but it was too late to save the manager, Steven Nelson, 55, of Copiague, who also was found unconscious in the basement.
- CCSP, 2008: Analyses of the effects of global change on human health and welfare and human systems. A Report by the U.S. Climate Change Science Program and the Subcommittee on Global Change Research. [Gamble, J.L. (ed.), K.L. Ebi, F.G. Sussman, T.J. Wilbanks, (Authors)]. U.S. Environmental Protection Agency, Washington, DC, USA. Web search 08/28/2010, original source: http://nepis.epa.gov/
- Health Effects of Carbon Dioxide - see "National Advisory Committee for Acute Exposure Guideline Levels (AEGLs) for Hazardous Substances; Proposed AEGL Values, Federal Register Document", http://www.epa.gov/EPA-TOX/2002/February/Day-15/t3774.htm note that these are proposed guidelines
- Carbon Dioxide CO2: Geologic Sequestration Health Effects: "Vulnerability Evaluation Framework
for Geologic Sequestration of Carbon Dioxide", US EPA, EPA430-R-08-009, July 2008, web search August 2010,original source: http://www.epa.gov/climatechange/emissions/downloads/VEF-Technical_Document_072408.pdf - Carbon Dioxide CO2: Geologic Sequestration, U.S. EPA, web search 08/28/2010, original source:
http://www.epa.gov/climatechange/emissions/co2_gs_tech.html- GTSP, 2006: Carbon Dioxide Capture and Geologic Storage: A Core Element of a A Global
Energy Technology Strategy to Address Climate Change (PDF, 37 pp., 6.05 MB, About PDF).
April 2006, JJ Dooley et al. Global Energy Technology Strategy Program (GSTP) - IPCC, 2005: Special Report on Carbon Dioxide Capture and Storage, Special Report of the
Intergovernmental Panel on Climate Change [Metz, Bert, Davidson, Ogunlade,
de Coninck, Heleen, Loos, Manuela, and Meyer, Leo (Eds.)]. Cambridge University Press, The
Edinburgh Building Shaftesbury Road, Cambridge CB2 2RU England
- GTSP, 2006: Carbon Dioxide Capture and Geologic Storage: A Core Element of a A Global
- Greenhouse Gas Overview: Carbon Dioxide: U.S. EPA, web search 08/28/2010, original source:
http://www.epa.gov/climatechange/emissions/co2.html - TABLE Z-1 LIMITS FOR AIR CONTAMINANTS, 1910.1000 TABLE Z-1 [PDF] OSHA standard for air contaminant limits (http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=STANDARDS&p_id=9992) - includes for CO2, Carbon dioxide.........| CAS No. 124-38-9 | 5000 ppm | 9000 mg/m3 limits for carbon dioxide as an air contaminant.
- In addition to citations & references found in this article, see the research citations given at the end of the related articles found at our suggested
CONTINUE READING or RECOMMENDED ARTICLES.
- Carson, Dunlop & Associates Ltd., 120 Carlton Street Suite 407, Toronto ON M5A 4K2. Tel: (416) 964-9415 1-800-268-7070 Email: info@carsondunlop.com. Alan Carson is a past president of ASHI, the American Society of Home Inspectors.
Thanks to Alan Carson and Bob Dunlop, for permission for InspectAPedia to use text excerpts from The HOME REFERENCE BOOK - the Encyclopedia of Homes and to use illustrations from The ILLUSTRATED HOME .
Carson Dunlop Associates provides extensive home inspection education and report writing material. In gratitude we provide links to tsome Carson Dunlop Associates products and services.