 Water Softener Alternatives
Water Softener Alternatives
Approaches to removing minerals from water other than a conventional water softener
- POST a QUESTION or COMMENT about non-salt-based water softening methods and water treatments for hard water
Water softener alternatives that do not use salt or other chlorides:
This article describes alternatives to conventional salt-based water softeners or water conditioners. Some of these alternatives work quite well at treating hard water (high in mineral content) but we warn that some other "water softener devices" often sold to homeowners are in fact ineffective.
InspectAPedia tolerates no conflicts of interest. We have no relationship with advertisers, products, or services discussed at this website.
- Daniel Friedman, Publisher/Editor/Author - See WHO ARE WE?
Other Water Softeners or Methods for Removing Hardness & Minerals from Water: Real vs. Fake Water Treatment Equipment
Effective Alternatives to Salt Based Water Treatment for Hard high-mineral content water
Other methods of water softening may have different potential effects on the septic system, as we discuss here.
Article Contents
- OTHER WATER SOFTENING METHODS
- CHELATING AGENTS & additives
- PRECIPITATING WATER SOFTENERS
- REVERSE OSMOSIS (RO) water purifiers
- IRON & MANGANESE REMOVAL
- POTASSIUM WATER SOFTENERS
- POLYPHOSPHATE TREATMENT SYSTEMS
- GREEN SAND & POTASSIIUM PERMANGANATE SYSTEMS
- CHLORINATION + CHARCOAL FILTRATION SYSTEMS
- ION EXCHANGERS & SALT BASED TREATMENT SYSTEMS
- QUESTIONABLE or INEFFECTIVE WATER SOFTENER & TREATMENT METHODS, MAGNETS, EPSOM SALTS
Chelating agents & chelating additives, treatments, or chelating systems for treating water
Chelating agents & chelating additives, treatments, or chelating systems for treating water high in minerals use chelating agents. Dow Chemical explains how chelating agents work to address minerals in water, various products, industrial processes, etc.
Soluble trace metals in aqueous systems exist as positively charged ions. Each of these ions has a fixed number of reactive sites.
Most metal ions have either four or six reactive sites. EDTA, DTPA, and HEDTA have six, eight, and six metal-complexing sites respectively, enabling one molecule to interact with all the reactive centers of a metal ion. NTA has four metal complexing sites, enabling one molecule to interact with the majority of the reactive centers of a metal ion. - Dow Chemical [1]
Reader Question: who can install a chelating cartridge to handle hard water?
I finally decided to do something about the hard well water and have ordered a chelating cartridge to be slipped into the stream after the particulate filter and before the big, blue reservoir water pressure tank.
Do you know of someone whom you would trust to look at the present arrangement and use their noggin when planning how to get the flow diverted out to a new cartridge holder and then back into the present line, providing a bypass and possibly even a takeoff to get water for the outside spigot out before it goes through the chelator?
I'd hate to see the job botched; it's such a neat piece of work as it stands.
I sure hope that you've heard good things about the chelation approach -- this uses citric acid to bind Ca++ and apparently (which makes sense) also can reverse calcium carbonate deposits already in place. It seemed to be a lot kinder to the septic tanks than a salt-based system. (Those have never made much sense to me, I'm afraid.). - E.O., New York
Reply: Discussion of chelating agents for home water softener treatment as an alternative to salt-based water softeners
Any licensed experienced plumber can recommend the installation location for your water treatment equipment and can handle the plumbing tasks themselves.
Chelating agents to bind various metals (iron, copper, manganese, calcium, and other metals occur naturally) that occur in water supply and in other raw materials that affect a wide variety of products, processes, and in mechanical systems have been in use for some time.
Much of the literature discusses food processing, personal care products, industrial applications, pharmaceuticals, and stabilization of other products affected by minerals in water, and treatment of boilers and similar equipment to clean or remove mineral deposits.
For example, Dow Chemical discusses the effectiveness of chelating agents, the varied uses of chelating agents and how chelating agents work in the company's product literature their VERSENE, VERSENEX, and VERSENOL Chelating Agents that Dow indicates are generically referred to as EDTA, HEDTA, DTPA and NTA.[1].
We don't have experience with the use of citric acid for those purposes.
However some authorities that citric acid, a weak organic acid, is effective in binding minerals in water. [2] Just how effective the filter system you are installing will be in handling the hardness of your water supply surely depends on the rated capacity of the filter (in gallons per day), your daily water usage, and the hardness of your water supply.
In your immediate area of Dutchess County, NY, the well water is very high in mineral content and also in iron. [2] [3]
We agree that avoiding dumping excessive salt into a septic system (a concern you expressed) is smart thinking.
A properly adjusted water softener should not be sending excessive salt to the septic drainfield, though indeed Gayman and others have reported on that concern. More septic system drainfield damage may be caused by flooding from excessive regeneration cycling or even a "stuck control" on a conventional water softener. Details are
But before buying and installing a specific water treatment device, we recommend that you test your water to determine the degree of hardness and that you compare your anticipated daily water usage rates with the treatment capacity rating of the chelating filter you are considering.
Precipitating water softeners
Precipitating water softeners using additives such as borax precipitate out minerals as a white sludge but I wouldn't recommend this equipment for residential use where clogging pipes and increased water alkalinity may be a problem.
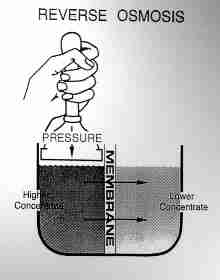
Reverse osmosis (RO) water purifiers
Reverse osmosis (RO) water purifiers (see sketch at page top) will also remove minerals from water leaving it soft. These systems do not discharge salt into the drain system, though they do discharge water.
We don't know (yet) which uses more discharge water - a water salt-based water softener or an RO system - I'll report that data here.
Culligan™ reports in their Water Softener Installation/Maintenance Guide that "The backwash interval is preset at the factory for 10 minutes which is adequate for most water supplies.
It is adjustable, however, for 5 to 30 minutes. It is recommended that backwash last just long enough so that the effluent from the drain line is clear. Backwash too long and water is wasted, not long enough and the tank becomes fouled with sediment."
Details about reverse osmosis are at REVERSE OSMOSIS WATER TREATMENT
See REVERSE OSMOSIS CONCENTRATE WASTE DISPOSAL for a discussion of the possible impact of reverse osmosis water treatment equipment's wastewater concentrate when it is disposed into a septic system.
Water Treatment Focused on Iron & Manganese Removal
Iron contaminants and high manganese contamination are often found along with water that is high in calcium and other mineral content. These treatment methods that focus on iron removal from well water: [16]
Potassium Chloride as a Salt Substitute in Water Softeners
During the water softener regeneration cycle, salty water (produced in the brine tank) washes calcium and other minerals out of the water softener while at the same time salt molecules are captured by the resin inside the water softener.
People who for health reasons need to minimize salt in their diet may consider a potassium chloride water softener treatment system as an alternative.
Watch out: different water softener settings may be required if you're using potassium chloride (KCl) to reduce the salt level in your softened water. Details are found
at WATER SOFTENER SALT SUBSTIUTE: POTASSIUM CHLORIDE.
Polyphosphate treatment of water supplies
Polyphosphate treatment can remove 0-3 ppm of soluble iron in water supplies.
Greensand Filters for Removing Iron & Sulphur in Water
Greensand Filters (using glauconite, a green clay mineral as ion exchange media glauconite, a media that is backwashed periodically using potassium permanganate) can remove 0-10 ppm of iron from water (and are great at removing sulphur odors as well). "Greensand" filters absorb soluble iron and manganese.
But check the pH of your water before using potassium permanganate. If your well water pH is less than 6.8 this approach doesn't work well.
Chlorination + charcoal filtration for Iron, manganese & odor removal in water
Chlorination + charcoal filtration: for higher levels of iron in water (not your case if your test was accurate) a chlorinator combined with charcoal filter can remove higher levels of iron and manganese dissolved in well water, and are also typically rated as 0-10 ppm.
The charcoal filter removes the residual [and potentially harmful carcinogenic thrhalomethanes (THM) produced by use of chlorine, residual chlorine, and thus and chlorine odors.
Because chlorine acts as a disinfectant, this design keeps the charcoal filter working longer by retarding bacterial growth. Periodically, however, the charcoal filter portion of the system has to be replaced.
Details are at CHLORINATORS & CHARCOAL FILTERS
Ion Exchangers for water treatment of soluble iron
Ion Exchangers (a water softener) can remove 0-10 ppm of soluble iron, but frankly water softeners are not designed primarily for that purpose. So often in high iron-content water homes I've examined, the professionals usually have installed one of the other iron removal methods upstream (ahead of) the water softener.
That also means you won't need to set the softener to use more salt than necessary to deal with hardness just because you're trying to remove iron from your water
Questionable or ineffective "water softener" water treatment methods
Some magic water softener equipment is sold such as magnets or other magic "catalytic" devices which claim to remove hardness (mineral ions) from the water by surrounding a water pipe with a magnet or other exciting ideas are simple junk science. The only "hardness" they remove is money from your wallet.
Epsom Salts and Septic Systems: Epsom salt is not sodium chloride. People who use Epsom salt baths, such as for a sore foot, are unlikely to discharge enough Epsom salts into a septic system for it to be measurable. Household use of Epsom salts should not be a concern for the septic system.
...
Continue reading at SCALE PREVENTION, WATER HEATER or select a topic from the closely-related articles below, or see the complete ARTICLE INDEX.
Or see these
Recommended Articles
Suggested citation for this web page
OTHER WATER SOFTENING METHODS at InspectApedia.com - online encyclopedia of building & environmental inspection, testing, diagnosis, repair, & problem prevention advice.
Or see this
INDEX to RELATED ARTICLES: ARTICLE INDEX to WATER TREATMENT SYSTEMS
Or use the SEARCH BOX found below to Ask a Question or Search InspectApedia
Ask a Question or Search InspectApedia
Try the search box just below, or if you prefer, post a question or comment in the Comments box below and we will respond promptly.
Search the InspectApedia website
Note: appearance of your Comment below may be delayed: if your comment contains an image, photograph, web link, or text that looks to the software as if it might be a web link, your posting will appear after it has been approved by a moderator. Apologies for the delay.
Only one image can be added per comment but you can post as many comments, and therefore images, as you like.
You will not receive a notification when a response to your question has been posted.
Please bookmark this page to make it easy for you to check back for our response.
IF above you see "Comment Form is loading comments..." then COMMENT BOX - countable.ca / bawkbox.com IS NOT WORKING.
In any case you are welcome to send an email directly to us at InspectApedia.com at editor@inspectApedia.com
We'll reply to you directly. Please help us help you by noting, in your email, the URL of the InspectApedia page where you wanted to comment.
Citations & References
In addition to any citations in the article above, a full list is available on request.
- [1] "Dow Chelating Agents, Effective, economical metal ion control VERSENE, VERSENEX, and VERSENOL Chelating Agents", The Dow Chemical Company, Midland, Michigan 48674, USA & Canada: 800-447-4369, Mexico: 95-800-447-4369, Europe: 800 3 694 6367* / 32 3 450 2240, website: www.versene.com web search 12/17/2011, original source: http://msdssearch.dow.com/PublishedLiteratureDOWCOM/ dh_003d/0901b8038003db82.pdf?filepath=versene/pdfs/noreg/113-01259.pdf&fromPage=GetDoc
- [2] Frank H. Verhoff (2005), "Citric Acid", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH [Wikipedia [3]]
- North Dakota Standards for Water Softeners, North Dakota General Authority Law, Chapter 62-04-08, Water Softener Units http://www.legis.nd.gov/information/acdata/pdf/62-04-08.pdf. "The objective of this chapter is to provide a standard of quality, capacity, and performance for water softener units. Water softener performance is to be based upon referee tests procedures described in section 62-04-08-09."
- [4] Culligan Mark 10 Water Softener 1994-1998 Installation and Operating Instructions (covering models manufactured after 1995) (1-96) 01881948.pdf available from www.culligan.com
- [6] "Commercial Water Softener Installation and Operating Instructions", IBC Filtration & Water Treatment Products (Australia) for commercial, industrial and residential application www.ibcwater.com.au (07) 3219 2233
- [7] "Non electric water softener, Installation and Operating Instructions", IBC Filtration & Water Treatment Products (Australia), op.cit.
- [8] ]"Water Softener Twin Tank Installation and Operating Instructions", IBC Filtration & Water Treatment Products (Australia), op.cit.
- Our recommended books about building & mechanical systems design, inspection, problem diagnosis, and repair, and about indoor environment and IAQ testing, diagnosis, and cleanup are at the InspectAPedia Bookstore. Also see our Book Reviews - InspectAPedia.
- In addition to citations & references found in this article, see the research citations given at the end of the related articles found at our suggested
CONTINUE READING or RECOMMENDED ARTICLES.
- Carson, Dunlop & Associates Ltd., 120 Carlton Street Suite 407, Toronto ON M5A 4K2. Tel: (416) 964-9415 1-800-268-7070 Email: info@carsondunlop.com. Alan Carson is a past president of ASHI, the American Society of Home Inspectors.
Thanks to Alan Carson and Bob Dunlop, for permission for InspectAPedia to use text excerpts from The HOME REFERENCE BOOK - the Encyclopedia of Homes and to use illustrations from The ILLUSTRATED HOME .
Carson Dunlop Associates provides extensive home inspection education and report writing material. In gratitude we provide links to tsome Carson Dunlop Associates products and services.

