 BOD5 Wastewater Testing Information
BOD5 Wastewater Testing Information
for Homeowners / Inspectors / Consultants
- POST a QUESTION or COMMENT about the significance of oxygen levels in wastewater and the definition of & standards for biological oxygen demand BOD5 used in Water Testing
What is BOD & how is it significant in water quality or potability testing?
This article describes Biological Oxygen Demand Testing or BOD5 wastewater testing, explains what the test involves, why it is used, and what it can tell us about private septic systems as well as the condition of public wastewater treatment facilities.
Understanding BOD or BOD5 tests of wastewater help diagnose drinking water quality problems in both private wells and community or public water supplies.To be clear, a BOD test is performed on wastewater to establish its level of treatment. It is not performed on drinking water. We include citations of standards & codes for wastewater testing. Graph at page top excerpted from the USGS Delzer and McKenzie publication described in this article.
InspectAPedia tolerates no conflicts of interest. We have no relationship with advertisers, products, or services discussed at this website.
- Daniel Friedman, Publisher/Editor/Author - See WHO ARE WE?
A Consumer's Guide to BOD5 5-day Biochemical Oxygen Demand used in wastewater testing
 Daniel Friedman, Isabel Sanchez Luna
Daniel Friedman, Isabel Sanchez Luna
The standard 5-day BOD5 test for biological oxygen demand in wastewater is used to evaluate the effectiveness of wastewater treatment by a public or private sewer or septic system.
If a septic system is not adequately treating its wastewater to remove biological pollutants the implication is that biological contaminants - a health hazard - are being discharged into the environment and thus potentially into local waterways and into drinking water supplies.
Our lab photo (left) illustrates a positive detection of total coliform in the left test tube and no coliform detected in the two samples at right. The test was performed using Lauril triptosa at dilution 1x10-4 as the culture media. Photo (left) I. Sanchez Luna.
Although there are numerous qualifications and potential test interferences that must be considered, the bottom line for a BOD5 test is that the acceptable level of wastewater BOD5 discharged to the environment must be at less than 0.2 mg/L (milligrams per liter), usually written as
< 0.2 mg/L
Delzer and McKenzie, writing for the USGS offer a succinct, clearly-worded technical explanations of the function of the biological oxygen demand test used to assay wastewater. The following excerpted quotations are from their USGS document [3]
Our lab photo (left) illustrates a positive detection of total coliform in all three test samples. Photo (left) I. Sanchez Luna.
The test for biochemical oxygen demand (BOD) is a bioassay procedure that measures the oxygen consumed by bacteria from the decomposition of organic matter (Sawyer and McCarty, 1978).
The change in DO concentration is measured over a given period of time in water samples at a specified temperature. Procedures used to determine DO concentration are described in NFM 6.2.
It is important to be familiar with the correct procedures for determining DO concentrations before making BOD measurements. BOD is measured in a laboratory environment, generally at a local or USGS laboratory.
 Watch out: the use of BOD to evaluate the effectiveness of wastewater treatment is focused on biological pollutants. It does not directly address many other contaminants that can be expected to appear in wastewater such as certain chemicals or heavy metals.
Watch out: the use of BOD to evaluate the effectiveness of wastewater treatment is focused on biological pollutants. It does not directly address many other contaminants that can be expected to appear in wastewater such as certain chemicals or heavy metals.
The BOD Wastewater Test Procedure
A sample of wastewater is collected in a clean container and delivered to a qualified testing laboratory (including labs provided by the USGS in the USA) within 24-hours of collection.
If the sample is not to be delivered immediately to a test facility it should be stored in a refrigerated or cooled container but kept above freezing. Ideal storage is at 1-4 degC.
Ideally the wastewater sample is delivered immediately to the test lab and the BOD test is performed immediately. If the sample cannot be delivered to the test laboratory within two hours it must be refrigerated and delivered to the test laboratory within 24-hours.
The sample quantity depends on the number and type of tests required; for municipal wastewater treatment systems generally a liter of sample is sufficient. For private lab testing of private septic systems, a smaller quantity is often acceptable. Check with your test lab. The manual gives this procedure for collecting a "grab sample" from a wastewater stream or from a nearby waterway:
When collecting a hand-dipped sample:
1. Grasp the sample container near the base on the downstream side of the bottle.
2. Plunge the bottle opening downward below the water surface. Avoid contact with the streambed during this process.
3. Allow the sample container to fill with the opening pointed slightly upward into the current.
4. Cap the container securely and protect the sample from light during transport to the laboratory for analysis.
As you will see on the water test form provided by your test laboratory, you will also need to record the date, time, and location from which the sample was collected.
An example of private use of this test method is a check on a pond or waterway located close to a private septic system suspected of leaching effluent directly into the waterway. Also
See SEPTIC DYE TEST PROCEDURE for additional site tests for septic effluent leakage out of the system.
Watch out: Close the wastewater sample and protect it from sunlight. Do not freeze the wastewater sample, and get it to the lab within 24-hours. Keeping a sample longer than 24-hours results in an invalid test.
Watch out: amateur collection of wastewaters for BOD testing risks an inaccurate result, as the field manual explains:
Bacteria are commonly associated with suspended sediment, which can vary spatially and temporally along a stream cross section (Britton and Greeson, 1989). Like suspended sediment, the oxygen-demanding compounds may not be equally distributed along a cross section.
Where possible, use the equal-width-increment or equal-discharge-increment procedures described in NFM 4 to collect a BOD sample representative of the stream cross section.[3]
Technical note: for private septic systems whose design includes a final disinfection stage the BOD procedure can produce erroneous results unless extra steps are taken by the test laboratory. Therefore it is important in private septic system treatment to inform the test laboratory of the type and condition of the septic system being tested.
In the U.S. the test laboratory follows detailed sample preparation and assay procedures specified in the NFM, National Field Manual for the Collection of Water-Quality Data; other countries use a very similar if not identical standard.
Typically a lab will prepare three different dilutions of the test sample to address the full anticipated range of possible biological oxygen demand. The sample(s) is/are prepared and then incubated at 20 deg C for five days.
At the end of the test interval the dissolved oxygen level (DO level) is measured to determine the BOD. By comparing the beginning oxygen level (DO at start of the test) with the ending oxygen level (DO at the end of the test), the BOD can be calculated. [1][2]
BOD5 Test Conclusion & Standard Formula & Calculation for Acceptable Level of Wastewater Treatment
In sum, at the end of the test interval the laboratory examines the quantity of dissolved oxygen consumed by biological entities in the sample. If the wastewater has been adequately treated the level of biological contaminants in the sample will be low enough that the BOD5 will be < 0.2 mg/L of liquid.
The general equation for the determination of a BOD5 value is:
BOD5 (mg/L) = D1 - D2 / P
D1= initial DO (dissolved oxygen level) of the sample,
D2= final DO of the sample at the end of the 5 day incubation period
P = decimal volumetric fraction of sample used [if the sample required dilution]
The Field Manual explains several possible causes of abnormal BOD5 readings such as poorly-cleaned lab glassware, de ionized water in the sample - factors that can increase the BOD5 reading, as well as interferences or improper lab ware that can cause abnormally low BOD5 readings.
Wastewater Treatment Level Terms & Definitions
Definition of BOD - Biological Oxygen Demand
The following definitions are excerpted from class notes provided by Nanyang Technological University.
"Biochemical oxygen demand (BOD) is defined as the amount of oxygen required by bacteria while stabilizing decomposable organic matter under aerobic conditions.
The BOD test is widely used to determine the pollution strength of domestic and industrial wastewaters in terms of the oxygen that they will require if discharged into natural watercourses in which aerobic conditions exist.
The test is one of the most important both in regulatory work and in studies designed to evaluate the purification capacity of receiving water bodies. Its disadvantage is the long time required by the test, generally taking 5 days." - Nanyang Technological University [4]
Definition of DO - Dissolved Oxygen
"All gases of the atmosphere are soluble in water to some degree. Oxygen is classified as poorly soluble, and its solubility is affected both by atmospheric pressure, and physical and chemical properties of water such as temperature, salinity, pollutants, etc. The solubility of atmospheric oxygen in fresh waters ranges from 14.6 mg/L at 0oC to about 7 mg/L at 35oC under 1 atm. of pressure.
Most of the critical conditions related to dissolved-oxygen deficiency, both in natural waters and biological wastewater treatment, occur during the warmer months when temperatures are high and solubility of oxygen is at a minimum. The low solubility of oxygen is a major factor limiting the purification capacity of natural waters.
In aerobic biological treatment processes, the limited solubility of oxygen is also of great importance, because it governs the rate at which oxygen will be absorbed by the medium and therefore the cost of aeration. Hence, DO analysis is a key test both in natural waters and water pollution control practice." - Nanyang Technological University [4]
Definition of CO or COD - Chemical Oxygen Demand
"Chemical oxygen demand (COD) is another parameter used widely to measure the pollution strength of domestic and industrial wastewaters. COD is defined as "total measurement of all chemicals in the water that can be oxidized" or as the amount of oxygen required to oxidize organic matter chemically.
Potassium dichromate (K2Cr2O7) is generally chosen for this purpose due to its strong chemical oxidizing capability.
Almost all organic compounds (except for ammonia, aromatic hydrocarbons, pyridine and their related compounds) can be oxidized by dichromate under heated acidic and AgSO4-catalysed conditions, equivalent to 95 – 100% of the theoretical values.
"One of the main limitations of the COD test is its inability to differentiate between biologically oxidizable and biologically inert organic matter. Nor can it provide any evidence of the biological decomposition rate that proceeds either in natural or man-made conditions.
The major advantage of COD test is the short time required for evaluation. The determination can be made in about 3 hr rather than the usual 5 days required for the measurement of BOD. - Nanyang Technological University [4]
"A COD test measures all organic carbon with the exception of certain aromatics (benzene, toluene, phenol, etc.) which are not completely oxidized in the reaction. Generally, COD is preferred to BOD for process control measurements because results are more reproducible and are available in just two hours rather than five days." [15]
Definition of TOC - Total Organic Carbon
"TOC measures the organic carbon concentration in the water and wastewater. The TOC test can be performed very rapidly (only several minutes) and conveniently (by TOC instrument) and is becoming more popular.
For a given wastewater, if a repeatable empirical relationship is established between its TOC and BOD or COD, then measurements in TOC can be used to estimate the accompanying BOD or COD. However, this relationship must be established independently for each set of conditions, such as at various points in a treatment process" - Nanyang Technological University [4]
Typical BOD5 Levels Found in Types of Wastewater or Sewage
| Common BOD5 Levels Found in Types of Wastewater or Sewage [4] | ||
|---|---|---|
| Raw Sewage BOD5 Levels | Weak Sewage Level | 110 mg/L |
| Medium Sewage | 220 mg/L | |
| Strong Sewage | 440 mg/L | |
| Sewage Levels Typical in Singapore | 300-350 mg/L | |
| Primary effluent | 150-200 mg/L | |
| Secondary effluent | 20-50 mg/L | |
| Tertiary effluent | 5-10 mg/L | |
| Raw reservoir water | Kranji | 5-15 mg/L |
| Upper Pierce | 3-7 mg/L | |
| Target BOD5 Level | < 0.2 mg/L | |
Notes to the table above
Data Source: excerpted & adapted from Laboratory Manual For Experiment Lab 2A-6(ENV) Wastewater Quality Analysis, Nanyang Technological University [4]
Typical Methods for Measuring BOD in Wastewater
- Above we described the standard 5-day incubation method for measuring BOD5 Levels Found in Types of Wastewater or Sewage and described in detail in the USGS manual [1][3]
Additional techniques for measuring BOD include
- An electronic probe that gives immediate oxygen level in water [4] Also see biosensors below.
- Bod-Bart™ - Biological Activity Reaction Tests (BART) developed and patented by Cullimore and Alford [4]. BOD-BART™ - based on enhanced respiration activity of the indigenous heterotrophic aerobic bacteria (HAB) inhabiting the sample.[5][6]
- Biosensors: biological sensing element with a transducer which produces a signal proportional to the analyte concentration. [5]
- Luminous
bacterial cells: immobilized chip method in which bacterial
bioluminescence which is caused by lux genes due reduction
or emission by physiological responses is measured and correlated to BOD. [5] - Ferricyanide-mediated BOD testing: ferricyanide has been used as e-acceptor instead of oxygen. [5]
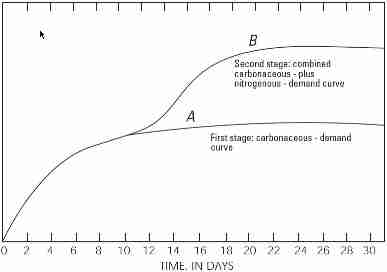
Two Stages of Decomposition of Biological Materials in Wastewater
The USGS graph repeated here illustrates the following [continuing to excerpt from the USGS document cited]:
There are two stages of decomposition in the BOD test: a carbonaceous stage and a nitrogenous stage (fig. 7.0-1).
- The carbonaceous stage, or first stage, represents that portion of oxygen demand involved in the conversion of organic carbon to carbon dioxide.
- The nitrogenous stage, or second stage, represents a combined carbonaceous plus nitrogeneous demand, when organic nitrogen, ammonia, and nitrite are converted to nitrate. Nitrogenous oxygen demand generally begins after about
6 days.
For some sewage, especially discharge from wastewater treatment plants utilizing biological treatment processes, nitrification can occur in less than 5 days if ammonia, nitrite, and nitrifying bacteria are present. In this case, a chemical compound that prevents nitrification should be added to the sample if the intent is to measure only the carbonaceous demand.
The results are reported as carbonaceous BOD (CBOD), or as CBOD5 when a nitrification inhibitor is used.
The standard oxidation (or incubation) test period for BOD is 5 days at 20 degrees Celsius (°C) (BOD5). The BOD5 value has been used
and reported for many applications, most commonly to indicate the effects of sewage and other organic wastes on dissolved oxygen in surface waters (see TECHNICAL NOTE). The 5-day value, however, represents only a portion of the total biochemical oxygen demand.
Twenty days is considered, by convention, adequate time for a complete biochemical oxidation of organic matter in a water sample, but a 20-day test often is impractical when data are needed to address an immediate concern.
- The BOD5 and CBOD5 tests have limited value by themselves in the assessment of stream pollution and do not provide
all of the relevant information to satisfy every study objective (Nemerow, 1974; Stamer and others, 1983; Veltz, 1984).
Additional analyses of water samples for chemical oxygen demand, fecal bacteria, and nutrients can aid in the interpretation of BOD5. - An ultimate carbonaceous BOD (CBODu) test is needed to obtain additional BOD information, and can be used for modeling DO regimes in rivers and estuaries (Hines and others, 1978; Stamer and others, 1983). Guidelines for the CBODu determination are described in Stamer and others (1979, 1983).
- Note that BOD results represent approximate stream oxygen demands because the laboratory environment does not reproduce ambient stream conditions such as temperature, sunlight, biological populations, and water movement.
Where does the "Five-Day" requirement for BOD5 Originate
Testing for an acceptable level of wastewater treatment needs a standard for comparison. The particular choice of five-days as the length of time used to measure oxygen demand is based on an interesting history cited by Delzer and McKenzie as a technical note:
A 5-day duration for BOD determination has no theoretical grounding but is based on historical convention.
Tchobanoglous and Schroeder (1985) provide the following background: "In a report prepared by the Royal Commission on Sewage Disposal in the United Kingdom at the beginning of the century, it was recommended that a 5-day, 18.3°C, BOD value be used as a reference in Great Britain.
These values were selected because British rivers do not have a flow time to the open sea greater than 5 days and average long-term summer temperatures do not exceed 18.3°C. The temperature has been rounded upward to 20°C, but the 5-day time period has become the universal scientific and legal reference."
BOD5 Wastewater Level Standards & Testing Research
- See additional BOD5 citaftions atReferences or Citations below.
- New York State Department of Health, Appendix 75-A Wastewater Treatment Standards - Individual Household Systems, [PDF] New York State Department of Health, 3 February 2010, retrieved 3/1/2010, original source: https://www.health.ny.gov/regulations/nycrr/title_10/part_75/appendix_75-a.htm
- Isabel Sanchez Luna is an environmental sciences student at the University of Guanajuato, Guanajuato, Mexico. Ms. Sanchez-Luna has interned at water and wastewater treatment facilities in the state of Guanajuato and has kindly agreed to assist in air and water quality studies & reports conducted under the aegis of InspectApedia.com beginning in 2012. She can be contacted through the website editor using the Contact Us links found on our web pages.
- [1] Water analisys - determination of the biochemical oxygen demand in natural, wastewaters (BOD5) and wastewaters treated - test method - web search 8/6/12, original source: http://www.conagua.gob.mx/CONAGUA07/Noticias/NMX-AA-028-SCFI-2001.pdf [Spanish Language copy on file as NMX-AA-028-SCFI-2001.pdf ]
- [2] Análisis de agua - determinación de la demanda bioquímica de oxígeno en aguas naturales, residuales (DBO5) y residuales tratadas - método de prueba (cancela a la nmx-aa-028-1981) - web search 8/6/12, original source: http://www.conagua.gob.mx/CONAGUA07/Noticias/NMX-AA-028-SCFI-2001.pdf [Spanish Language copy on file as NMX-AA-028-SCFI-2001.pdf ]
- [3] Delzer, G.C., and McKenzie, S.W., November 2003, Five-day biochemical oxygen demand: U.S. Geological Survey Techniques of Water-Resources Investigations, book 9, chap. A7 (3d ed.), section 7.0, accessed 8/6/2012 from http://pubs.water.usgs.gov/twri9A/., original source http://water.usgs.gov/owq/FieldManual/Chapter7/NFMChap7_2_BOD.pdf, [copy on file as NFMChap7_2_BOD.pdf]
* The USGS is a science organization that provides impartial information on the health of our ecosystems and environment, the natural hazards that threaten us, the natural resources we rely on, the impacts of climate and land-use change, and the core science systems that help us provide timely, relevant, and usable information. - http://www.usgs.gov/ - [4] "Laboratory Manual
For
Experiment Lab 2A-6(ENV)
Wastewater Quality Analysis", Nanyang Technological University
School Of Civil And Environmental Engineering, July 19, 2004, retrieved 8/6/2012, original source: http://www.cee.ntu.edu.sg/AboutCEE/Facilities/EnvLab/Documents
/CV2701%202A-6.pdf, [ copy on file as NanyangTU_CV2701 2A-6.pdf] - [5] Mayur Milan Kale and Indu Mehrotra, "Rapid Determination of Biochemical Oxygen
Demand", retrieved 8/6/12, original source: http://www.waset.org/journals/ijcee/v1/v1-1-3.pdf [copy on file as Kayle_Determine_BOD_v1-1-3.pdf] Abstract:
Abstract
Biochemical Oxygen Demand (BOD) is a measure of the oxygen used in bacteria mediated oxidation of organic substances in water and wastewater. Theoretically an infinite time is required for complete biochemical oxidation of organic matter, but the measurement is made over 5-days at 20 0C or 3-days at 27 0C test period with or without dilution. Researchers have worked to further reduce the time of measurement.
The objective of this paper is to review advancement made in BOD measurement primarily to minimize the time and negate the measurement difficulties. Survey of literature review in four such techniques namely BOD-BARTTM, Biosensors, Ferricyanidemediated approach, luminous bacterial immobilized chip method. Basic principle, method of determination, data validation and their advantage and disadvantages have been incorporated of each of the methods.
In the BOD-BARTTM method the time lag is calculated for the system to change from oxidative to reductive state. BIOSENSORS are the biological sensing element with a transducer which produces a signal proportional to the analyte concentration. Microbial species has its metabolic deficiencies.
Co-immobilization of bacteria using sol-gel biosensor increases the range of substrate. In ferricyanidemediated approach, ferricyanide has been used as e-acceptor instead of oxygen. In Luminous bacterial cells-immobilized chip method, bacterial bioluminescence which is caused by lux genes was observed.
Physiological responses is measured and correlated to BOD due to reduction or emission.
There is a scope to further probe into the rapid estimation of BOD. Keywords—BOD, Four methods, Rapid estimation. - [6] Johnston L., Cullimore R and Singh K.,. (1990), Field Trials of the BOD-BART system™ for the Rapid Determination of Biochemical Oxygen Demand in Secondary and Tertiary Effluents. Droycon Bioconcepts Inc, Regina, Saskatchewan, and University of New Brunswick, Fredricton, New Brunswick.
Question: so how do I find the septic tank
(Dec 27, 2012) connie said:
i do not know where my septic is.i just bought this home and the people before me can not be found.the house was built in 1997 it is now 2013 when does it need to be treated?i have a big front yard and back yard,and i want to build a deck.
(Mar 18, 2014) Dwight Dove said:
I am buying 5 acres of land and know that a septic tank is on the property but do not know where it is. How can I find it?
Reply:
Dwight, have you reviewed the suggestions in the septic tank location article above?
If you knew nothing about a site you'd look at reasonable locations where a tank could fit and where there are not mature trees, away from a well, etc. as suggested above.
Question: septic tank distance from the house?
(Apr 8, 2014) Natalie said:
does anyone know how far your septic tank needs to be away from your house?
Reply:
Sure Natalie,
Near the top of this article click on the "Click to Show or Hide Related Topics"
then click on the article titled
CLEARANCE DISTANCES, SEPTIC SYSTEM
for the details you need
Question:
5/12/14 Al said:
i bought a house recently,on my disclosure it says i have city sewer,but i discovered from local plumber with the use of video camera that my basement toilet drained into a septic tank which was full and backing up into my basement.plumber could not find a clean out for it but was able to locate part of it under a cement slab and the addition of my house.what to do .do i leave it alone or do i somehow get it removed.house was built in the 50's.
Reply:
Al,
It's not uncommon for an older home to suffer from confused public records about its connection to public sewer for all or part of its wastewater drainage.
A septic tank is always full in normal operation. When there's a backup it's because of some other failure: a failed drainfield or a blocked pipe. When the property owner is facing significant repair costs such as that of a new drainfield, that's the time to go ahead and connect to the now-available public sewer instead. At that time one would properly abandon the septic tank by having it pumped out and filled-in.
...
Continue reading at BIOMAT FORMATION & SEPTIC LIFE if you are looking for its location, or select a topic from the closely-related articles below, or see the complete ARTICLE INDEX.
Or see these
Recommended Articles
- HOW TO OPEN a SEPTIC TANK if you have found the septic tank an dare inspecting or servicing the septic tank
Suggested citation for this web page
BOD WASTEWATER TEST at InspectApedia.com - online encyclopedia of building & environmental inspection, testing, diagnosis, repair, & problem prevention advice.
Or see this
INDEX to RELATED ARTICLES: ARTICLE INDEX to SEPTIC SYSTEMS
Or use the SEARCH BOX found below to Ask a Question or Search InspectApedia
Ask a Question or Search InspectApedia
Questions & answers or comments about the significance of oxygen levels in wastewater and the definition of & standards for biological oxygen demand BOD5 used in Water Testing.
Try the search box just below, or if you prefer, post a question or comment in the Comments box below and we will respond promptly.
Search the InspectApedia website
Note: appearance of your Comment below may be delayed: if your comment contains an image, photograph, web link, or text that looks to the software as if it might be a web link, your posting will appear after it has been approved by a moderator. Apologies for the delay.
Only one image can be added per comment but you can post as many comments, and therefore images, as you like.
You will not receive a notification when a response to your question has been posted.
Please bookmark this page to make it easy for you to check back for our response.
IF above you see "Comment Form is loading comments..." then COMMENT BOX - countable.ca / bawkbox.com IS NOT WORKING.
In any case you are welcome to send an email directly to us at InspectApedia.com at editor@inspectApedia.com
We'll reply to you directly. Please help us help you by noting, in your email, the URL of the InspectApedia page where you wanted to comment.
Citations & References
In addition to any citations in the article above, a full list is available on request.
- [7] Sawyer C., McCarty P., Parkin G. (2006)“Chemistry for Environmental Engineering and Sciences”, Tata McGraw-Hill Publishing Company, fifth edition.
- [8] Logan, B.E. and Wagenseller G.A.(1993). The HBOD test: a new method for determining biochemical oxygen demand. Water Environ. Res. (65-7), p: 862-868.
- [9] APHA, Standard Methods for the Examination of Waters and Wastewater. 20th Edn.(2005). American Public Health Association, Washington, DC.
- [10] Cullimore, R., (1999), Biological Activity Reaction Tests- A simple yet effective method for monitoring Biochemical Oxygen Demand.BARTTM testers. Practical Atlas for Bacerial Identification, Lewis/CRC Press, Droycon Bioconcepts Inc. 315 Dewdney Ave. Regina, Saskatchewan, Canada S4N 0E7.
- [11] Karube, I., Matsunaga, T., Mitsuda, S., Suzuki, S., (1977.), Microbial Biosensors Biotechnol. Bioeng. (19), p.1535–1547.
- [12] Rastogi S, Rathee P, Saxena T.K., Mehra N.K., Kumar R.(2002) BOD analysis of industrial effluents: 5 days to 5 min, Current Applied Physics (3),p-191–194.
- [13] Hikuma M., Suzuki, H., Yasuda, T., Karube, I., Suzuki, S., (1979). Amperometric estimation of BOD by using living immobilized yeast, Eur. J. Appl. Microbiol. Biotechnol. (8), 289–297.
- [14] Toshifumi Sakaguchi , Yasunori Morioka , Masahiro Yamasaki , Junpei Iwanaga , Kazuhiko Beppu, Hideaki Maeda, Yasutaka Morita , Eiichi Tamiya. (2006), Rapid and onsite BOD sensing system using luminous bacterial cells-immobilized chip, Biosensors and Bioelectronics (19),p- 115-121.
- [15] Environmental Leverage, Inc., "What is the difference between BOD, COD or TOC? Why do I have to measure them?", 812 Dogwood Drive Suite A, North Aurora, IL 60542, Tel: 1-630-906-9791, Email: support@environmentalleverage.com, retrieved 8/6/12, original source: http://www.environmentalleverage.com/BOD%20vs%20COD.htm, [copy on file as BOC_ COD_EnvLev.pdf]
- Our recommended books about building & mechanical systems design, inspection, problem diagnosis, and repair, and about indoor environment and IAQ testing, diagnosis, and cleanup are at the InspectAPedia Bookstore. Also see our Book Reviews - InspectAPedia.
- In addition to citations & references found in this article, see the research citations given at the end of the related articles found at our suggested
CONTINUE READING or RECOMMENDED ARTICLES.
- Carson, Dunlop & Associates Ltd., 120 Carlton Street Suite 407, Toronto ON M5A 4K2. Tel: (416) 964-9415 1-800-268-7070 Email: info@carsondunlop.com. Alan Carson is a past president of ASHI, the American Society of Home Inspectors.
Thanks to Alan Carson and Bob Dunlop, for permission for InspectAPedia to use text excerpts from The HOME REFERENCE BOOK - the Encyclopedia of Homes and to use illustrations from The ILLUSTRATED HOME .
Carson Dunlop Associates provides extensive home inspection education and report writing material. In gratitude we provide links to tsome Carson Dunlop Associates products and services.

