Toxicity of Oxygen Gas Exposure, O2
Toxicity of Oxygen Gas Exposure, O2
Poisoning Symptoms, Oxygen Gas Exposure Limits, and Links to Toxic Gas Testing Procedures
- POST a QUESTION or COMMENT about Oxygen gas exposure limits and toxicity for humans
Effects of exposure to abnormal levels of oxygen gas:
This document discusses the toxicity and exposure limits for exposure to oxygen gas O2. We also describe the normal levels of oxygen in the atmosphere and include a chart of the history of oxygen levels on earth.
We give references and explanation regarding toxicity of oxygen, based on literature search and obtained from the U.S. government and expert sources. This text may assist readers in understanding these topics. However it should by no means be considered complete nor authoritative. Seek prompt advice from your doctor or health/safety experts if you have any reason to be concerned about exposure to toxic gases.
InspectAPedia tolerates no conflicts of interest. We have no relationship with advertisers, products, or services discussed at this website.
O2 POISONING SYMPTOMS - Oxygen Gas poisoning symptoms
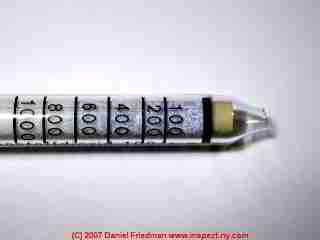
Recommendations for tools and methods for detecting gases in buildings or outdoors
The photo shows a Drager colorimetric gas detection tube (also called a "color detector tube") used to test levels of a very wide range of specific gases in air. In an indoor air test (in our laboratory) this particular detector was not being used to measure oxygen, but rather carbon dioxide.
As the blue-stained portion of the tube shows, we found that the CO2 level was about 600ppm which is typical of indoor air and is an acceptable and safe level.
Colorimetric gas detection tubes such as those sold by Drager (or Draeger), Gastec, (two that we use predominantly) and by Kitagawa, and pumps from Drager, Gastec, Komyo Rikagaku Kitagawa, and RAE all work on a similar principle: a measured volume of gas (or air) is drawn through a tube which contains chemicals which change in color in response to the presence of a specific target gas (or range of gases) present in the sample.
By knowing the volume of gas or air sampled, the amount of color change read on a linear scale on the colorimetric gas detection tube can be translated into a very accurate measurement of level of gas present, described in percentage of the total air or in parts per million (PPM).
Other specialized gas detection methods include use of solid state circuity, CMS chips, and special instruments which may be designed to give a quick alarm or a reading in PPM for specific gases. Other gas and air monitoring equipment use pumps which collect and insert a specific volume of air into a vacuum container for later analysis.
We've found that for typical field use, the colorimetric gas detector tube method is extremely convenient and very accurate, and it presents minimal requirements for instrument calibration.
What happens if a human breathes too much oxygen rather than too little?
Since we've discussed displacement of and reduction of the level of oxygen by an increase in CO2 or by dangerous carbon monoxide CO levels indoors in occupied buildings, (see links for these topics at the ARTICLE INDEX the bottom of this article ) let's take a look at the toxicity of oxygen itself and at what happens if the mix of gases in air changes in the opposite direction, increasing rather than decreasing the level of oxygen above normal amounts.
Where does this occur? Two examples of exposure of humans to unusually high levels of oxygen occur in "oxygen bars" and in hyperbaric medicine.
Technical note on oxygen toxicity: What about too much oxygen in air:
While high levels, even 100% oxygen are used for medical purposes in some cases (hyperbaric oxygen patients breathe 100% O2 at pressures above 2 to 2.4 ATM for 40-60 minutes),breathing high levels of oxygen can be toxic for humans and other animals. (Toxicity of excessive oxygen will depend on the oxygen level, the duration of exposure (breathing it), and individual characteristics).
But breathing high O2 air too long can produce toxic free radicals, producing effects that can be observed clinically in the human lungs or central nervous system. The current recommended limits of oxygen exposure are up to 24 hours with 100% O2, and up to 48 hours with 60% O2. OSHA does not have a permissible exposure limit (PEL) for oxygen.
What are the symptoms of exposure to elevated levels of oxygen?
The symptoms of excessive oxygen exposure include the following: (some or all of these might occur)
- Dizziness can occur on exposure to high oxygen levels
- Coughing and irritations of the throat can occur on exposure to high oxygen levels
- Difficulties with vision or visual anomalies can occur at very high O2concentrations
What are the medical effects or symptoms of prolonged exposure to 100% oxygen?
If a person (say a medical patient undergoing hyperbaric treatment at high levels of oxygen and at increased pressure) is breathes 100% oxygen for more than a day (24 hours) s/he may show the symptoms we listed just above, and also the following:
- General physical weakness or a sense of exhaustion or fatigue can occur at very high O2concentrations
- The patient may experience pain joint and muscle pain
- The high oxygen prolonged exposure individual may experience numbness and tingling in arms and legs (like when your leg "falls asleep")
- Heart palpitations may occur
- The individual may complain of a headache
- Some patients complain of nasal congestion or (perhaps similarly-caused ear clogging, congestion, and pain, perhaps caused by swelling of the mucous membranes
- On 24 hour exposure to 100% oxygen additional medical symptoms include the development of fever, nausea and vomiting, and a (corresponding) loss of appetite
- Experts also cite the obvious hazard of freezing-burns if someone comes in contact with liquid oxygen.
Reader Question: At what level does Oxygen become toxic ?
05/04/2015 Anonymous said:
What is the ppm at which oxygen becomes toxic?
Reply:
Anon, OSHA has not set a PEL for Oxygen.
Bert originally described that CNS toxicity occured at oxygen pressures of > 3 ATA, it may however, occur at lower pressures if exposure is prolonged. Note that this is at three atmospheres of pressure. Though early manifestations are variable, twitching of perioral and small muscles of the hand is a fairly constant feature. Intense peripheral vasoconstriction due to hyperoxia and diaphragmatic twitching can result in facial pallor and ‘cogwheel’ breathing, respectively4,7.
Continuation of exposure can lead to vertigo and nausea followed by altered behaviour, clumsiness, and finally convulsions. The convulsions are usually tonic-clonic, and the patient has no memory of the crisis4,8. A neurogenic pulmonary oedema concomitant with the convulsions has also been reported .
The factors aggravating the CNS toxicity are raised pCO2, stress, fatigue, cold, and dietary deficiency of trace elements such as selenium, zinc and magnesium1,4,10. CNS toxicity is mainly due to oxidation and polymerisation of -SH groups of enzymes leading to their inactivation, which in turn results in cellular damage.
... Pulmonary effects of oxygen toxicity can occur after a prolonged exposure to oxygen > 0.5 ATA. Symptoms appear after a latent period whose duration decreases with increase in pO2 . In normal humans, the first signs of toxicity appear after 10 hours of oxygen at 1 ATA. (Chawla (2001) - According to Patel et als (JIACM 2003; 4(3): 234-7)
- Chawla A, Lavania AK. Oxygen toxicity. Medical Journal Armed Forces India 2001; 57: 131-3.
- Donald KW. Oxygen poisoning in man (I,II). BMJ 1947; 667- 72, 712-7.
- Edmonds C, Lowry C, Pennefather J. Oxygen toxicity. In : Edmond C, Lowry C, Pennefather J, Editors. Diving and Subaquatic Medicine. Oxford; Butterworth-Heinemann. 1992; 241-56.
- Patel, Dharmeshkumar N, Ashish Goel**, SB Agarwal, Praveenkumar Garg, Krishna K Lakhani, "Oxygen Toxicity", Journal, Indian Academy of Clinical Medicine, Vol. 4 No. 3, July-September 2003, JIACM 2003; 4(3): 234-7, retrieved 4 May 2015, original source: http://medind.nic.in/jac/t03/i3/jact03i3p234.pdf
What is the Normal Level of Oxygen in Air?
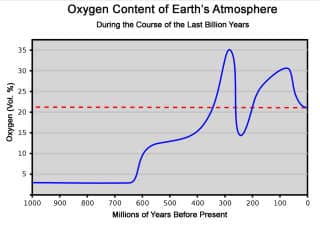
The normal level of oxygen in outdoor air is just under 21%. This graph from Wikipedia (ret. 2018 07 07) illustrates the history of variation level of oxygen in air on the earth. Before there were people the level was as low as 5%, and its peak was 35%.
[Click to enlarge any image]
Original source of the Wikipedia graph above: LordToran derivative work: WolfmanSF (talk) - Sauerstoffgehalt-1000mj.svg translated from the original German.
Dieses Diagramm stellt die Sauerstoffkonzentration in der Erdatmosphäre im Lauf der letzten Jahrmilliarde dar. Als Referenz zeigt die gestrichelte rote Linie die heutige Konzentration von 21% an. ...
This graph represents the oxygen concentration in the Earth's atmosphere over the last few billion years. For reference, the dashed red line indicates today's concentration of 21%.
It should be noted that the periods in which past fluctuations in the O2 concentration occurred can often only be estimated relatively roughly. Therefore, the diagram does not claim to be exact, but can give only a rough overview of the evolution at the level of geological periods.
This diagram is based on averages or matches from several publications - cited in the original article - Ed.
Explanation of the curve:
After the outgassing of oxygen from the oceans had already begun in the Precambrian, the oxygen content jumped abruptly from about 3% to 12% at the beginning of the Cambrian, as finally all oxygen sinks were saturated. At about the same time, the Cambrian explosion started.
During the Silurian and Devonian plants conquered the land, while the animal kingdom was still confined almost exclusively to the water.
This triggered another rapid and continuous increase in oxygen concentration. In the following Carboniferous it had reached 35%, which favored gigantism, especially in insects. Amphibians and the first reptiles have also settled in the country.
Massive volcanic activity caused not only a drop in oxygen content to 15% at the Permian-Triassic transition, but also the largest mass extinction of the Earth's history.
The oxygen concentration recovered over a long period and reached 26% in the middle of the Jurassic, probably even 30% in the Cretaceous. During this time lived the largest dinosaurs.
The end of the Cretaceous is marked by an impact event and a climate change with mass extinction. 40 million years ago, the oxygen content was only 23% and had reached its current level of 21% 25 million years ago. Since then, it has been constant, except for fluctuations in the per thousand range.
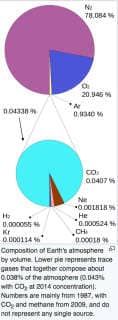
The nice pair of pie charts below, also from Wikipedia portrays the mix of gases in air. Op. Cit. ret- 7/7/2018
[Click to enlarge any image]
...
Continue reading at GAS EXPOSURE LIMITS & STANDARDS or select a topic from the closely-related articles below, or see the complete ARTICLE INDEX.
Or see these
Recommended Articles
- GAS DETECTION INSTRUMENTS
- GAS EXPOSURE EFFECTS, TOXIC
- CONVERT PPM to % CONCENTRATION To convert between % and ppm
Suggested citation for this web page
OXYGEN - O2 at InspectApedia.com - online encyclopedia of building & environmental inspection, testing, diagnosis, repair, & problem prevention advice.
Or see this
INDEX to RELATED ARTICLES: ARTICLE INDEX to GAS HAZARDS in BUILDINGS
Or use the SEARCH BOX found below to Ask a Question or Search InspectApedia
Ask a Question or Search InspectApedia
Questions & answers or comments about Oxygen gas exposure limits and toxicity for humans.
Try the search box just below, or if you prefer, post a question or comment in the Comments box below and we will respond promptly.
Search the InspectApedia website
Note: appearance of your Comment below may be delayed: if your comment contains an image, photograph, web link, or text that looks to the software as if it might be a web link, your posting will appear after it has been approved by a moderator. Apologies for the delay.
Only one image can be added per comment but you can post as many comments, and therefore images, as you like.
You will not receive a notification when a response to your question has been posted.
Please bookmark this page to make it easy for you to check back for our response.
Our Comment Box is provided by Countable Web Productions countable.ca
Citations & References
In addition to any citations in the article above, a full list is available on request.
- OSHA provides these literature citations for this symptom list (which we paraphrased from OSHA documents):
International Chemical Safety Cards (WHO/IPCS/ILO): Oxygen.
Pohanish, R.P. (editor): Oxygen. In, Sittig's Handbook of Toxic and Hazardous Chemicals and Carcinogens, Fourth Ed., Vol. 2. Norwich, NY: Noyes Publications, William Andrew Publishing, 2002, pp. 1771-1772. www.osha.gov/dts/chemicalsampling/data/CH_259105.html and www.vspn.org/vspnsearch/aow/oxygentoxicity.htm Oxygen toxicity Oxygen Toxicity Compendium of Continuing Educational Practice, Veternarian 21[4]:341-351 Apr'99 Review Article 67 References, by Steven Mensack, VMD & Robert Murtaugh, DVM, MS, at the Department of Clinical Science, Tufts University School of Veterinary Medicine, North Grafton, MA
"Room Air Appears To Do Less Brain Damage Than Pure Oxygen", Doctor's Guide, September 18, 1998, which we found at www.pslgroup.com/dg/B01A6.htm This article points out that while oxygen is an important component of treatment for certain medical emergencies (perhaps heart attack), after oxygen levels reached normal levels in the blood, continuing to administer pure oxygen actually was damaging the metabolic mechanism in the brain. [close paraphrasing] - In addition to citations & references found in this article, see the research citations given at the end of the related articles found at our suggested
CONTINUE READING or RECOMMENDED ARTICLES.
- Carson, Dunlop & Associates Ltd., 120 Carlton Street Suite 407, Toronto ON M5A 4K2. Tel: (416) 964-9415 1-800-268-7070 Email: info@carsondunlop.com. Alan Carson is a past president of ASHI, the American Society of Home Inspectors.
Thanks to Alan Carson and Bob Dunlop, for permission for InspectAPedia to use text excerpts from The HOME REFERENCE BOOK - the Encyclopedia of Homes and to use illustrations from The ILLUSTRATED HOME .
Carson Dunlop Associates provides extensive home inspection education and report writing material. In gratitude we provide links to tsome Carson Dunlop Associates products and services.